Credit: Courtesy: Anniina Vihervaara
New research reveals how cancer cells endure stress and survive. Publishing in Molecular Cell, an international research team identified mechanisms that human and mouse cells use to survive heat shock and resume their original function – and even pass the memory of the experience of stress down to their daughter cells.
Lead author Anniina Vihervaara, Assistant Professor in Gene Technology at KTH Royal Institute of Technology, says the results provide insight into the mechanisms that coordinate transcription in cells, which potentially could make a vital contribution in disease research.
The researchers examined how embryonic fibroblast cells and cancer cells responded when subjected to heat shock at a temperature of 42C, using advanced technology to monitor the process of transcription across genes and their regulatory regions. Heat shock causes acute proteotoxic stress due to misfolding and aggregation of proteins. To adjust and maintain stability, stressed cells reduce protein synthesis and increase expression of chaperones that help other proteins to maintain their correct configuration. The heat shock response and protein misfolding are involved in many diseases, including cancer, Huntington’s and Alzheimer’s.
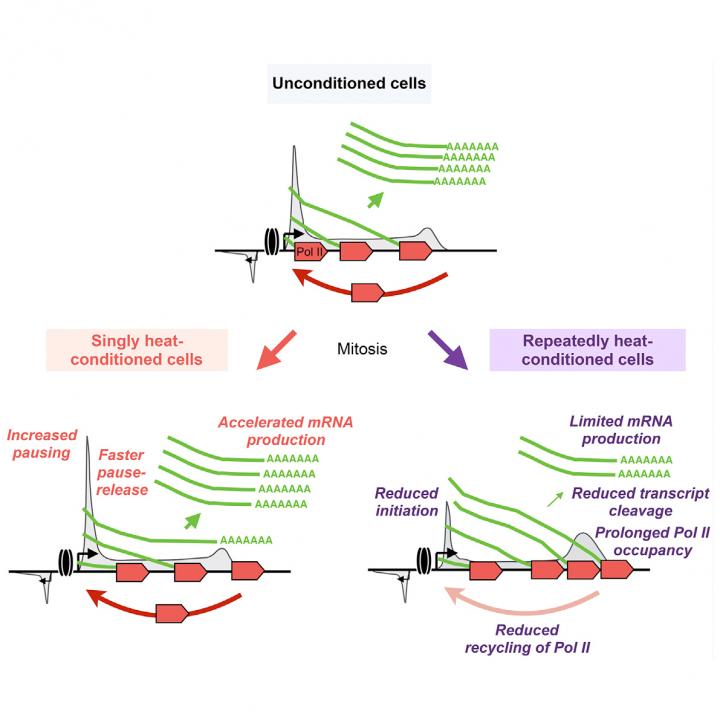
The mouse embryonic cells used in the study were sensitive to stress and did not survive prolonged or repeated heat shocks, but a model of cancer cells fared better – they survived multiple episodes of stress and maintained their rate of proliferation.
“Cancer cells are professional survivors and that’s what we saw in this study,” Vihervaara says.
How they did so proved remarkable, she says. The heat shock completely changes the transcriptional program of cells. Within minutes the cells switch to survival mode, inducing hundreds of genes, while repressing thousands more, she says.
Cells retain the repressed genes in a rapidly activable state by pausing transcription machinery at the early part of the gene. Once the stress is relieved, the cells recover within hours by allowing the transcription to continue, and the cell returns to executing its cell-type-specific transcription program.
Yet the revelations didn’t stop there. The researchers observed how the cell transmits the transcriptional memory of its reaction to its daughter cells, that is, those cells that bud from cell division.
“Autophagocytosis-related genes in cells were activated faster if the parental cells had experienced stress. These genes help the cell to get rid of misfolded proteins,” she says. “Cancer cells also slowed RNA processing at the ends of the genes to reduce the burden for protein production.”
Vihervaara’s group at KTH’s joint research center, Science for Life Laboratory (SciLifeLab), uses a technique (Precision Run-On sequencing) that monitors the progression of transcription at genes and enhancers at a nucleotide-resolution across the genome, followed by advanced data-analyses.
“Our aim is to bring this technical knowledge to physiological settings, where it can contribute to medical research,” she says. “But first we need to understand the mechanisms of transcriptional reprogramming in model cell lines before we can understand them in physiological settings.”
###
Media Contact
Anniina Vihervaara
[email protected]
Related Journal Article
http://dx.



