New biomaterials developed at the University of Bayreuth prevent colonization by bacteria and fungi, but at the same time proactively assist in the regeneration of human tissue. These nanostructured materials are based on spider silk proteins.
Credit: Images: Gregor Lang.
New biomaterials developed at the University of Bayreuth eliminate risk of infection and facilitate healing processes. A research team led by Prof. Dr. Thomas Scheibel has succeeded in combining these material properties which are highly relevant to biomedicine. These nanostructured materials are based on spider silk proteins. They prevent colonization by bacteria and fungi, but at the same time proactively assist in the regeneration of human tissue. They are therefore ideal for implants, wound dressings, prostheses, contact lenses, and other everyday aids. The scientists have presented their innovation in the journal Materials Today.
It is a widely underestimated risk of infection: Microbes settling on the surfaces of objects indispensable in medical therapy or for quality of life generally. Gradually, they form a dense, often invisible biofilm that cannot be easily removed, even by cleaning agents, and which often is resistant against antibiotics and antimycotics. Bacteria and fungi can then migrate into the adjacent tissue of the organism. As a result, they not only interfere with various processes of healing, but can even cause life-threatening infections.
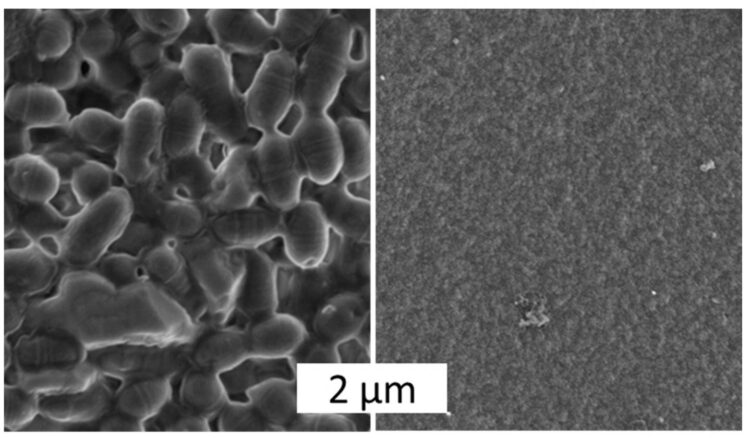
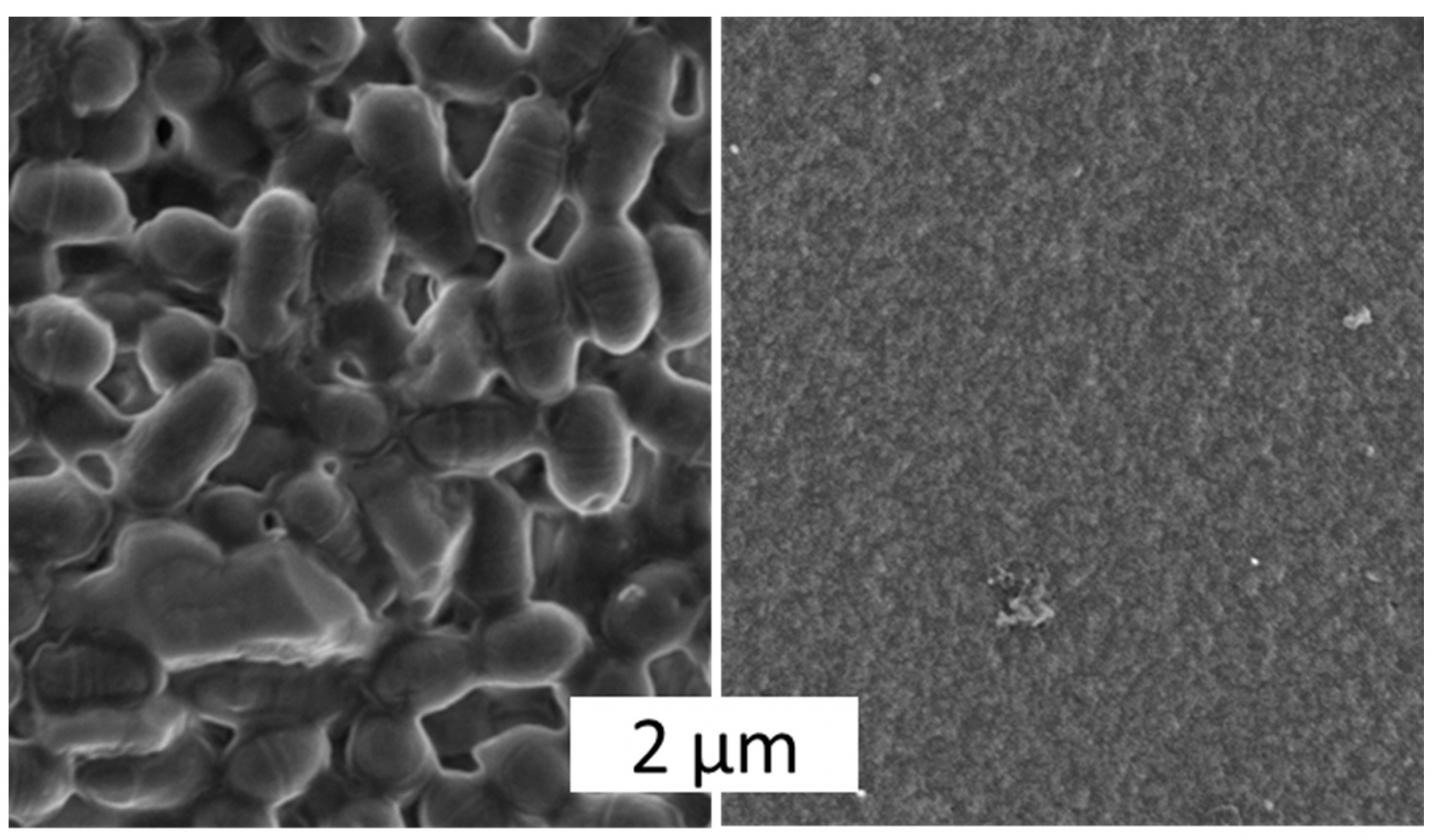
With a novel research approach, University of Bayreuth scientists have now found a solution to this problem. Using biotechnologically produced spider silk proteins, they have developed a material that prevents the adhesion of pathogenic microbes. Even streptococci, resistant to multiple antibacterial agents (MRSA), have no chance of settling on the material surface. Biofilms growing on medical instruments, sports equipment, contact lenses, prostheses, and other everyday objects may therefore soon be history.
Moreover, the materials are designed to simultaneously aid the adhesion and proliferation of human cells on their surface. If they can be used for e.g. wound dressings, skin replacement, or implants, they proactively support the regeneration of damaged or lost tissue. In contrast to other materials that have previously been used to regenerate tissue, the risk of infection is intrinsically eliminated. Microbial-resistant coatings for a variety of biomedical and technical applications are thus set to become available in the near future.
The Bayreuth researchers have so far successfully tested the microbe-repellent function on two types of spider silk materials: on films and coatings that are only a few nanometres thick and on three-dimensional hydrogel scaffolds which can serve as precursors for tissue regeneration. “Our investigations to date have led to a finding that is absolutely ground-breaking for future research work. In particular, the microbe-repellent properties of the biomaterials we have developed are not based on toxic, i.e. not cell-destroying, effects. The decisive factor rather lies in structures at the nanometre level, which make the spider silk surfaces microbe-repellent. They make it impossible for pathogens to attach themselves to these surfaces”, explains Prof. Dr. Thomas Scheibel, who is the Chair of Biomaterials at the University of Bayreuth.
“Another fascinating aspect is that nature has once again proven to be the ideal role model for highly advanced material concepts. Natural spider silk is highly resistant to microbial infestation and the reproduction of these properties in a biotechnological way is a break-through”, adds Prof. Dr.-Ing. Gregor Lang, one of the two first authors and head of the research group of Biopolymer Processing at the University of Bayreuth.
In the Bayreuth laboratories, spider silk proteins were specifically designed with various nanostructures in order to optimize biomedically relevant properties for specific applications. Once again, the networked research facilities on the Bayreuth campus have proven their worth. Together with the Bavarian Polymer Institute (BPI), three other interdisciplinary research institutes of the University of Bayreuth were involved in this research breakthrough: the Bayreuth Centre for Material Science & Engineering (BayMAT), the Bayreuth Centre for Colloids & Interfaces (BZKG), and the Bayreuth Centre for Molecular Biosciences (BZKG).
###
Research cooperation: In the study now published in Materials Today, the team from the University of Bayreuth worked together with research partners at Saarland University and Gießen University.
Research funding: The research work at the University of Bayreuth was funded by the German Research Foundation (DFG) within the framework of the collaborative research centres TRR 225 (“From the Basics of Biofabrication to Functional Tissue Models”) and SFB 840 (“From Particulate Nanosystems to Mesotechnology”).
Media Contact
Thomas Scheibel
[email protected]
Original Source
https:/
Related Journal Article
http://dx.