Rutgers researchers are developing materials to help grow human tissues
Credit: Madison Madison Godesky
Rutgers biomedical engineers have developed a “bio-ink” for 3D printed materials that could serve as scaffolds for growing human tissues to repair or replace damaged ones in the body.
The study was published in the journal Biointerphases.
Bioengineered tissues show promise in regenerative, precision and personalized medicine; product development; and basic research, especially with the advent of 3D printing of biomaterials that could serve as scaffolds, or temporary structures to grow tissues.
Hyaluronic acid, a natural molecule found in many tissues throughout the body, has many properties ideal for creating customized scaffolds, but lacks the durability required. The Rutgers engineers use modified versions of hyaluronic acid and polyethylene glycol to form a gel that is strengthened via chemical reactions and would serve as a scaffold.
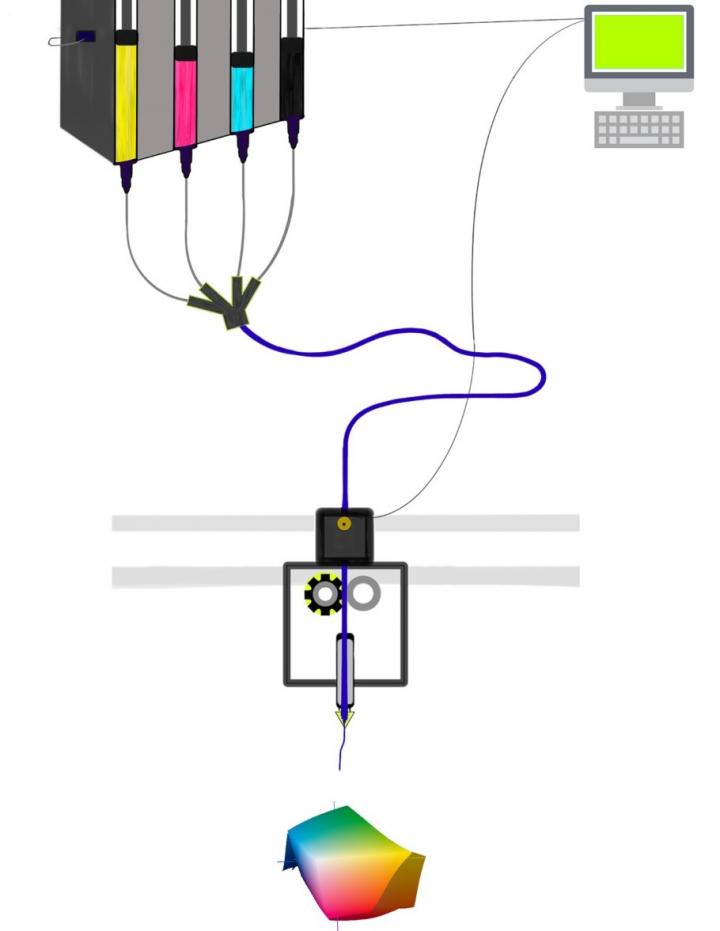
“Instead of an ink color for an inkjet printer, we want the mixture to have properties that are right for specific cells to multiply, differentiate and remodel the scaffold into the appropriate tissue,” said senior author David I. Shreiber, a professor who chairs the Department of Biomedical Engineering in the School of Engineering at Rutgers University-New Brunswick. “We focus on the stiffness of the gel and scaffold binding sites that cells can latch onto.”
Groups of cells in the body generally make their own support structures, or scaffolds, but scientists can build them from proteins, plastics and other sources, according to the National Institutes of Health.
Shreiber and lead author Madison D. Godesky, who earned a doctorate at Rutgers, envisioned a system where hyaluronic acid and polyethylene glycol serve as the basic “ink cartridges” for 3D printing. The system would also have other ink cartridges featuring different cells and ligands, which serve as binding sites for cells. The system would print gel scaffolds with the right stiffness, cells and ligands, based on the type of tissue desired.
“Both the stiffness and the binding sites provide important signals to cells,” Godesky said. “What especially distinguishes our work from previous studies is the potential to control the stiffness and ligands independently through combinations of inks.”
###
Media Contact
Todd Bates
[email protected]
848-932-0550
Original Source
https:/
Related Journal Article
http://dx.




