Tough, bioengineered peptide is a major advance in brain tumor imaging that could enable more precise surgical removal.In a breakthrough that could have wide-ranging applications in molecular medicine, Stanford University researchers have created a bioengineered peptide that enables imaging in lab mice of medulloblastomas, among the most devastating of malignant childhood brain tumors.
The researchers altered the amino acid sequence of a cystine knot peptide – or knottin – derived from the seeds of the squirting cucumber, a plant native to Europe, North Africa and parts of Asia. Peptides are short chains of amino acids that are integral to cellular processes; knottin peptides are notable for their stability and resistance to breakdown.
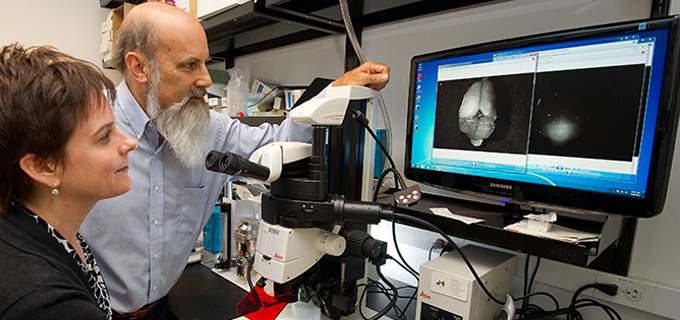
The team used its invention as a “molecular flashlight” to distinguish tumors from surrounding healthy tissue. After injecting the bioengineered knottin into the bloodstreams of mice with medulloblastomas, the researchers found that the peptide stuck tightly to the tumors and could be detected using a high-sensitivity digital camera.
The findings are described in a study released Aug. 12 in the Proceedings of the National Academy of Sciences.
“Researchers have been interested in this class of peptides for some time,” said Jennifer Cochran, an associate professor of bioengineering and a senior author of the study. “They’re extremely stable. For example, you can boil some of these peptides or expose them to harsh chemicals, and they’ll remain intact.”
That makes them potentially valuable in molecular medicine. Knottins could be used to deliver drugs to specific sites in the body or, as Cochran and her colleagues have demonstrated, as a means of illuminating tumors.
For treatment purposes, it’s critical to obtain accurate images of medulloblastomas. In conjunction with chemotherapy and radiation therapy, the tumors are often treated by surgical removal. And it can be difficult to remove them while leaving healthy tissue intact because their margins are often indistinct.
“With brain tumors, you really need to get the entire tumor and leave as much unaffected tissue as possible,” Cochran said. “These tumors can come back very aggressively if not completely removed, and their location makes cognitive impairment a possibility if healthy tissue is taken.”
The researchers’ molecular flashlight works by recognizing a biomarker on human tumors. The bioengineered knottin is conjugated to a near-infrared imaging dye. When injected into the bloodstreams of a strain of mice that develop tumors similar to human medullublastomas, the peptide attaches to the brain tumors’ integrin receptors – sticky molecules that aid in adhesion to other cells.
But while the knottins stuck like glue to tumors, they were rapidly expelled from healthy tissue. “So the mouse brain tumors are readily apparent,” Cochran said. “They differentiate beautifully from the surrounding brain tissue.”
The new peptide represents a major advance in tumor-imaging technology, said Melanie Hayden, a neurosurgeon at the Stanford Brain Tumor Center and a lead author of the paper. The most common extant technique employs a high-contrast dye that is injected intravenously shortly before or during an operation. Tumors absorb some of the dye and can be identified on a magnetic resonance imaging scan.
“But that has limitations,” Hayden said. “When you’re using dye and an MRI scan, you’re basically working off a snapshot. And the brain can sometimes shift during an operation, so there’s always the possibility you may not be precisely where you want to be. The great advantage of this new approach is that you’re illuminating the tumor in real time – you’re seeing it directly under your scope instead of relying on an image that was taken earlier.” An important next step will be to translate these results from mice to human patients.
Though the team’s research focused on medulloblastomas, Hayden said it’s likely the new knottins could prove useful in addressing other cancers.
“We know that integrins exist on many types of tumors,” she said. “The blood vessels that tumors develop to sustain themselves also contain integrins. So this has the potential for providing very detailed, real-time imaging for a wide variety of tumors.”
And imaging may not be the only application for the team’s engineered peptide.
“We’re very interested in related opportunities,” Cochran said. “We envision options we didn’t have before for getting molecules into the brain.” In other words, by substituting drugs for dye, the knottins might allow the delivery of therapeutic compounds directly to cranial tumors – something that has proved extremely difficult to date because of the blood/brain barrier, the mechanism that makes it difficult for pathogens, as well as medicines, to traverse from the bloodstream to the brain.
“We’re looking into it now,” Cochran said.
A little serendipity was involved in the peptide’s development, said Sarah Moore, a recently graduated bioengineering PhD student and another lead author of the study. Indeed, the propinquity of Cochran’s laboratory to co-author Matthew Scott’s lab at Stanford’s James H. Clark Center catalyzed the project. “Our labs are next to each other,” Moore said. “We had the peptide, and Matt had ideal models of pediatric brain tumors – mice that develop tumors in a similar manner to human medulloblastomas. Our partnership grew out of that.”
Scott, professor of bioengineering and of developmental biology, credits the design of the Clark Center as a contributor to the project. The building is home to Stanford’s Bioengineering Department, a collaboration between the School of Engineering and the School of Medicine, and Stanford Bio-X, an initiative that encourages communication among researchers in diverse scientific disciplines.
“So in a very real sense, our project wasn’t an accident,” Scott said. “In fact, it’s exactly the kind of work the Clark Center was meant to foster. The lab spaces are wide and open, with very few walls and lots of glass. We have a restaurant that only has large tables — no tables for two, so people have to sit together. Everything is designed to increase the odds that people will meet and talk. It’s a form of social engineering that really works.”
Scott said he is gratified by the collaboration that led to the team’s breakthrough and observed that the peptide has proved a direct boon to his own work. About 15 percent of Scott’s mice develop the tumors requisite for medulloblastoma research. The problem, he said, is that the cancers are cryptic in their early stages.
“By the time you know the mice have them, many of the things you want to study – the genesis and development of the tumors – are past,” Scott said. “We needed ways to detect these tumors early, and we needed methods for following the steps of tumor genesis.”
Ultimately, Scott concluded, the development of the new peptide can be attributed to Stanford’s long-established traditions of openness and relentless inquiry.
“You find not just a willingness, but an eagerness to exchange ideas and information here,” Scott said. “It transcends any competitive instinct, any impulse toward proprietary thinking. It is what makes Stanford – well, Stanford.”
The Stanford Center for Children’s Brain Tumors at Lucile Packard Children’s Hospital is supporting ongoing work by the group to translate the new technology into patient care. Additional funding came from the Wallace H. Coulter Foundation, the V Foundation for Cancer Research, the James S. McDonnell Foundation, the Stanford Cancer Institute, the National Science Foundation, a Stanford University graduate fellowship, a Siebel Scholars fellowship, a Gerald J. Lieberman fellowship, the California Institute of Regenerative Medicine and the Stanford Child Health Research Institute.
Other Stanford co-authors were postdoctoral scholar Jamie Bergen, PhD; medical student Yourong Sophie Su; and life science research assistant Helen Rayburn.
Story Source:
The above story is reprinted from materials provided by Stanford University News, Glen Martin.