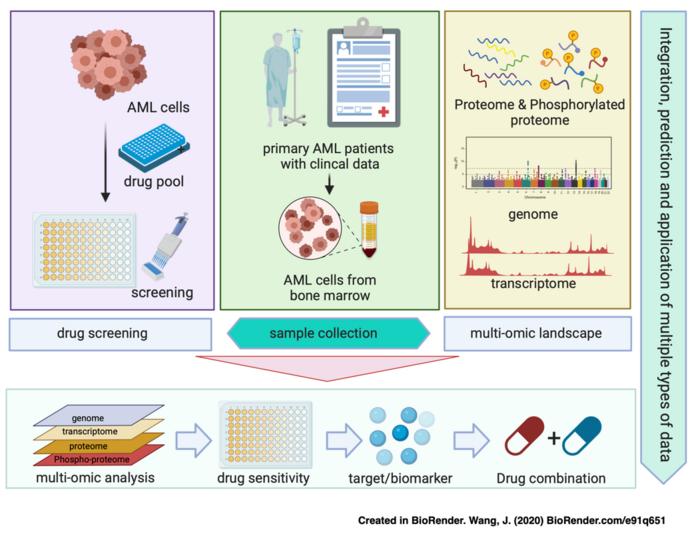
Acute myeloid leukaemia (AML) is a complex and diverse group of malignancies characterized by an accumulation of immature myeloid cells within the bone marrow, which leads to a substantial decrease in the number of mature blood cells. This type of leukaemia accounts for approximately 28% of all leukaemia cases, presenting a significant challenge to oncologists and researchers alike, particularly due to its poor prognosis, with a bleak five-year survival rate hovering around just 30.5%. The current therapeutic landscape for AML is far from adequate, emphasizing the urgent need for innovative approaches in the understanding and treatment of this aggressive disease.
Recent advances in quantitative proteomics have begun to highlight the intratumoral heterogeneity present within Western AML patients, revealing divergent protein expression profiles and phosphorylation patterns. Despite these pioneering findings, integrative analyses combining drug sensitivity data with comprehensive multi-omics approaches are sorely lacking, particularly those that account for the nuances of AML molecular characteristics and their associations with drug responses. Moreover, there is an alarming scarcity of information regarding the proteomic landscape of AML in Asian populations, which is critical for the development of targeted therapies that address these unique patient cohorts.
In a groundbreaking study, a research team led by Professor Jia Li and colleagues from the Shanghai Institute of Materia Medica collaborated with Professor Jianmin Yang’s team from the Naval Medical University to undertake a thorough genomic, proteomic, and phosphoproteomic analysis encompassing 101 Chinese AML samples. This study is particularly noteworthy as it also included an expansive in vitro drug sensitivity analysis evaluating the efficacy of 77 different drugs. Through proteome-based unsupervised clustering, the study identified three distinct subtypes of AML, each exhibiting unique molecular signatures and clinical outcomes, thus providing a more nuanced understanding of the disease.
The importance of selecting the correct subtype is underscored by the study’s implementation of consensus clustering methodology, which robustly indicated that three subtypes were statistically more valid than the options of two or four subtypes. However, intriguingly, no distinct prognostic differences emerged when comparing these three entities. Nonetheless, the research team uncovered a critical finding: patients classified within the subtype S-I exhibited higher measurable residual disease (MRD) levels post-therapy, indicative of a compromised clearance efficiency that could have profound implications for treatment strategies.
Additionally, the implications of allogenic hematopoietic stem cell transplantation (Allo-SCT) were critically assessed in the context of these cellular subtypes. The treatment did not appear to confer any survival advantages for patients within subtype S-I, which raised significant concerns regarding the efficacy of current protocols for this subgroup. Conversely, patients classified as S-II and S-III demonstrated a considerable survival benefit from Allo-SCT, suggesting that this form of intervention may represent a pivotal therapeutic avenue for these specific patients.
In a further effort to unravel the intricate relationship between drug sensitivity and the molecular characteristics of AML, the research team conducted extensive high-throughput screening. They initially screened over 2,200 compounds, followed by an additional round involving 285 compounds that were tested against primary tumour cells extracted from 56 AML patients. This comprehensive profiling against a panel of 77 inhibitors, which included both clinical chemotherapy drugs and targeted agents such as kinase inhibitors and epigenetic modifiers, offered invaluable insights into the potential for drug repurposing and combination strategies in the realm of AML treatment.
One particularly compelling outcome from this screening was the discovery of a positive correlation between higher expression levels of ALDH3A2 and enhanced sensitivity to the chemotherapy agent cytarabine. Using a combination of an ALDH3A2 inhibitor, disulfiram, alongside cytarabine treatment, the researchers observed significant synergistic effects across various AML cell lines. This promising discovery raises the possibility that disulfiram could substantially improve the therapeutic efficacy of cytarabine for numerous cell types, showcasing the potential benefits of leveraging multi-omics data to inform and transform current treatment paradigms.
Moreover, the multi-omics analysis yielded critical insights into the mechanisms underlying resistance to Acalisib, a PI3K inhibitor, within AML cell populations. Correlation analyses revealed that high levels of PDK activation were directly associated with decreased sensitivity to Acalisib. Interestingly, co-targeting PDK with the inhibitor GSK2334470 in combination with Acalisib demonstrated a marked enhancement of anticancer effects, emphasizing the importance of integrated treatment strategies that consider the specificity of each molecular pathway involved in the disease progression.
The findings from this extensive multi-omic analysis not only deepen our understanding of the complexities inherent in AML but also open the door to the development of more tailored and effective therapeutic strategies. By linking the molecular characteristics of AML subtypes with their clinical outcomes, researchers can forge a pathway towards personalized medicine, transforming the way we approach diagnosis and treatment of this formidable disease.
In conclusion, this study stands at the forefront of AML research, offering a glimpse into the future of oncological treatment where individualized therapies based on robust molecular data may become the standard. Integrative analyses such as this one hold the promise of reshaping our understanding of cancer biology and ultimately improving survival rates and outcomes for patients afflicted by acute myeloid leukaemia.
Subject of Research: Acute Myeloid Leukaemia (AML)
Article Title: Integrative Proteogenomic and Pharmacological Landscape of Acute Myeloid Leukaemia
News Publication Date: November 2024
Web References: http://dx.doi.org/10.1016/j.scib.2024.11.020
References: Referenced study data and methodologies were utilized throughout the article.
Image Credits: ©Science China Press
Keywords: Acute Myeloid Leukaemia, Proteogenomics, Drug Sensitivity, Multi-Omics, Personalized Medicine, Molecular Characteristics, Allogenic Stem Cell Transplantation.