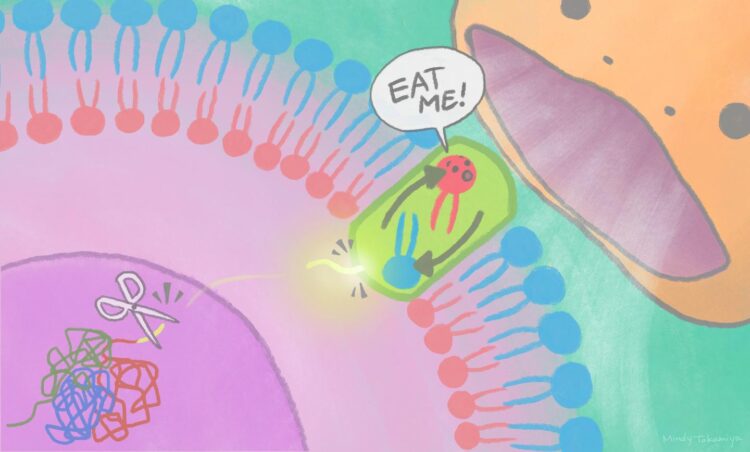
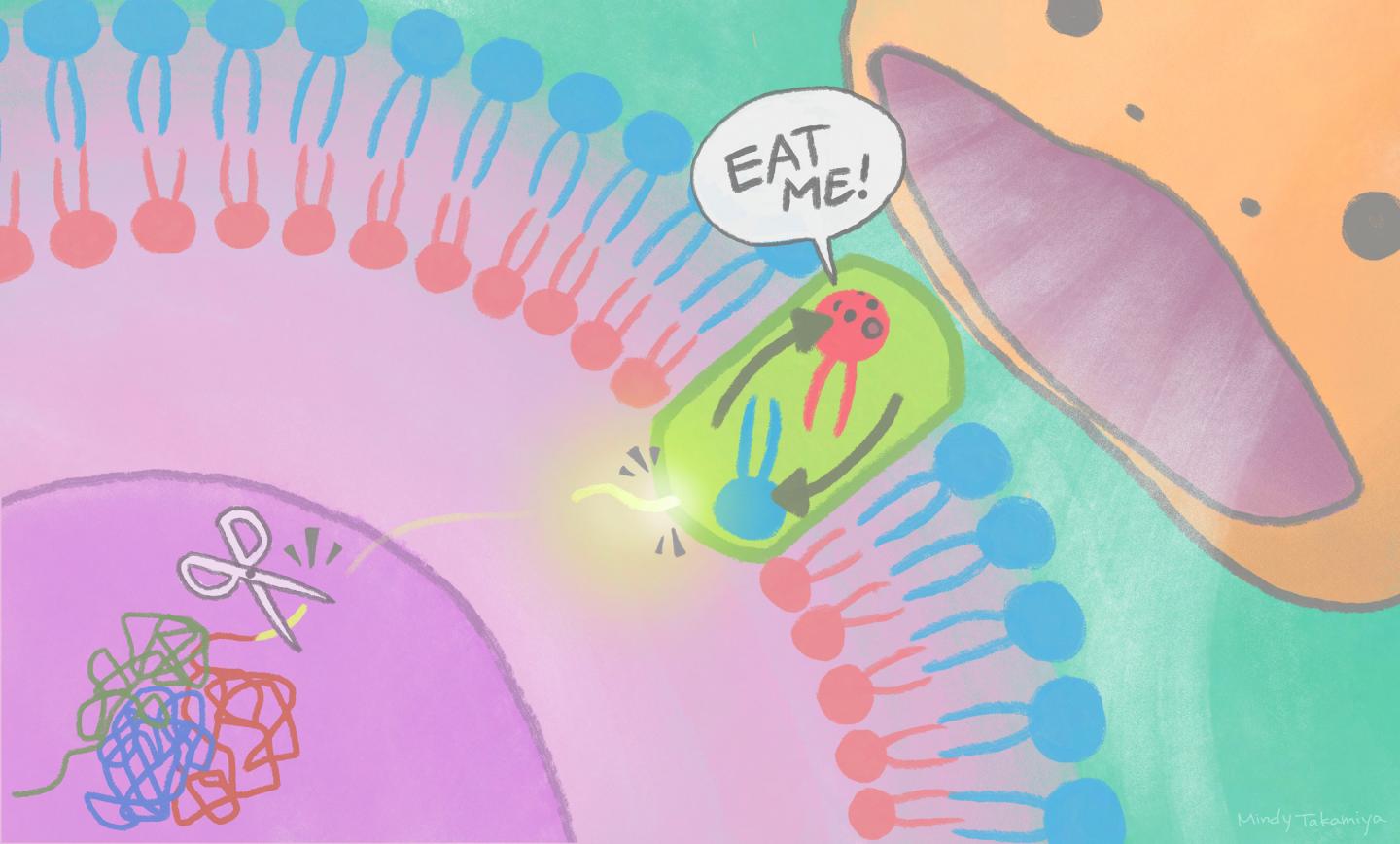
An ‘eat-me’ signal displayed on cell surfaces requires activation of a lipid-scrambling protein by a nuclear protein fragment
Credit: Mindy Takamiya/Kyoto University iCeMS
Scientists at the Institute for Integrated Cell-Material Sciences (iCeMS) and colleagues in Japan have revealed molecular mechanisms involved in eliminating unwanted cells in the body. A nuclear protein fragment released into the cytoplasm activates a plasma membrane protein to display a lipid on the cell surface, signalling other cells to get rid of it. The findings were published in the journal Molecular Cell.
“Every day, ten billion cells die and are engulfed by blood cells called phagocytes. If this didn’t happen, dead cells would burst, triggering an auto-immune reaction,” explains iCeMS biochemist Jun Suzuki, who led the study. “It is important to understand how dead cells are eliminated as part of our body’s maintenance.”
Scientists already know that dead cells display an ‘eat me’ signal on their surface that is recognized by phagocytes. During this process, lipids are flipped between the inner and outer parts of the cell membrane via a variety of proteins called scramblases. Suzuki and his team have already identified several of these lipid-scrambling proteins, but some of their activation mechanisms have been unclear.
To solve this, the team used an array of screening approaches to study the scrambling protein called Xkr4. The broad aim was to single out the genes that are active during cell death and to specifically zoom in on Xkr4 and its associated proteins to understand how they interact.
“We found that a nuclear protein fragment activates Xkr4 to display the ‘eat me’ signal to phagocytes,” says iCeMS cell biologist Masahiro Maruoka, the first author of the study.
Specifically, the scientists found that cell death signals lead to a nuclear protein, called XRCC4, getting cut by an enzyme. A fragment of XRCC4 leaves the nucleus, activating Xkr4, which forms a dimer: the linking of identical pieces into configurations. Both XRCC4 binding and dimer formation are necessary for Xkr4 to ultimately transfer lipids on the cell surface to alert phagocytes.
Xkr4 is only one of the scrambling proteins. Others are activated much faster during cell death. The team now wants to understand when and why the Xkr4 pathway is specifically activated. Since it is strongly expressed in the brain, it is likely important for brain function. “We are now studying the elimination of unwanted cells or compartments in the brain to understand this process further,” says Maruoka.
###
DOI: 10.1016/j.molcel.2021.02.025
About Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS):
At iCeMS, our mission is to explore the secrets of life by creating compounds to control cells, and further down the road to create life-inspired materials.
https:/
For more information, contact:
I. Mindy Takamiya/Mari Toyama
[email protected]
Media Contact
Mindy Takamiya
[email protected]
Related Journal Article
http://dx.