An old dog with new tricks
Credit: Alan Herbert
In a paper just published in the Journal for the Immunotherapy of Cancer (doi:10.1136/jitc-2020-001712), Dr Herbert, Head of Discovery at InsideOutBio, asks whether cancer cells stiff-arm immune cells just like rugby players push off defenders from the other team. He describes the nanoscale machinery used by tumors to fend off the immune system. The molecules involved are capable of flexing and extending as needed, just like a rugby player’s arm. The tumor’s stiff-arm tactic limits the effectiveness of current immunotherapeutics, such as checkpoint inhibitors. These treatments only work once the initial tackle is made, allowing the defender to recover the ball and go on to score.
Cancer cells make many abnormal proteins. The tumors they form should be easy for the immune system to find and destroy. Yet the cancer cells carry on uninterrupted. The mechanism they use to fend off immune cells is based on a defense system that reaches right back to the dawn of multi-cellular organisms. The system tags normal cells that belong differently from those that should not be there. It originally evolved as a defense against bacteria and disease-causing viruses. It is based on a set of more than 30 proteins, referred to as the complement system, which has been studied by scientists for the last 130 years. The complement system places an “I belong” tag on normal cells to prevent the immune system from attacking them. Cancer cells exploit this mechanism to fend off the immune system. They do so by producing the “I belong” tag in excess. They need to “Super-Self” themselves to hide all the abnormal proteins they have on their surface. They are then able to run past the immune cell defenders without being tackled.
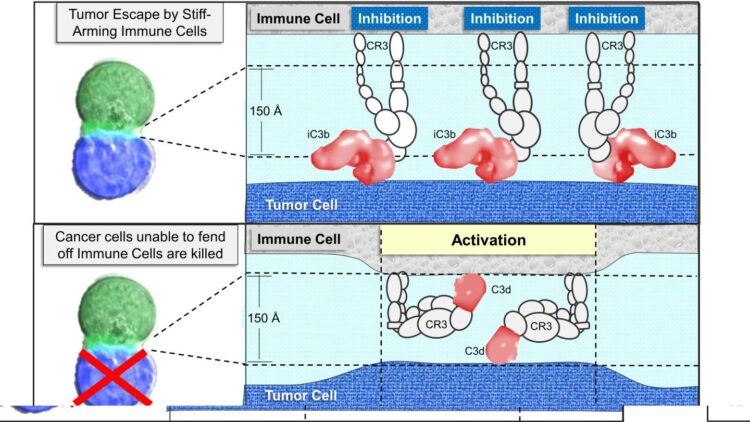
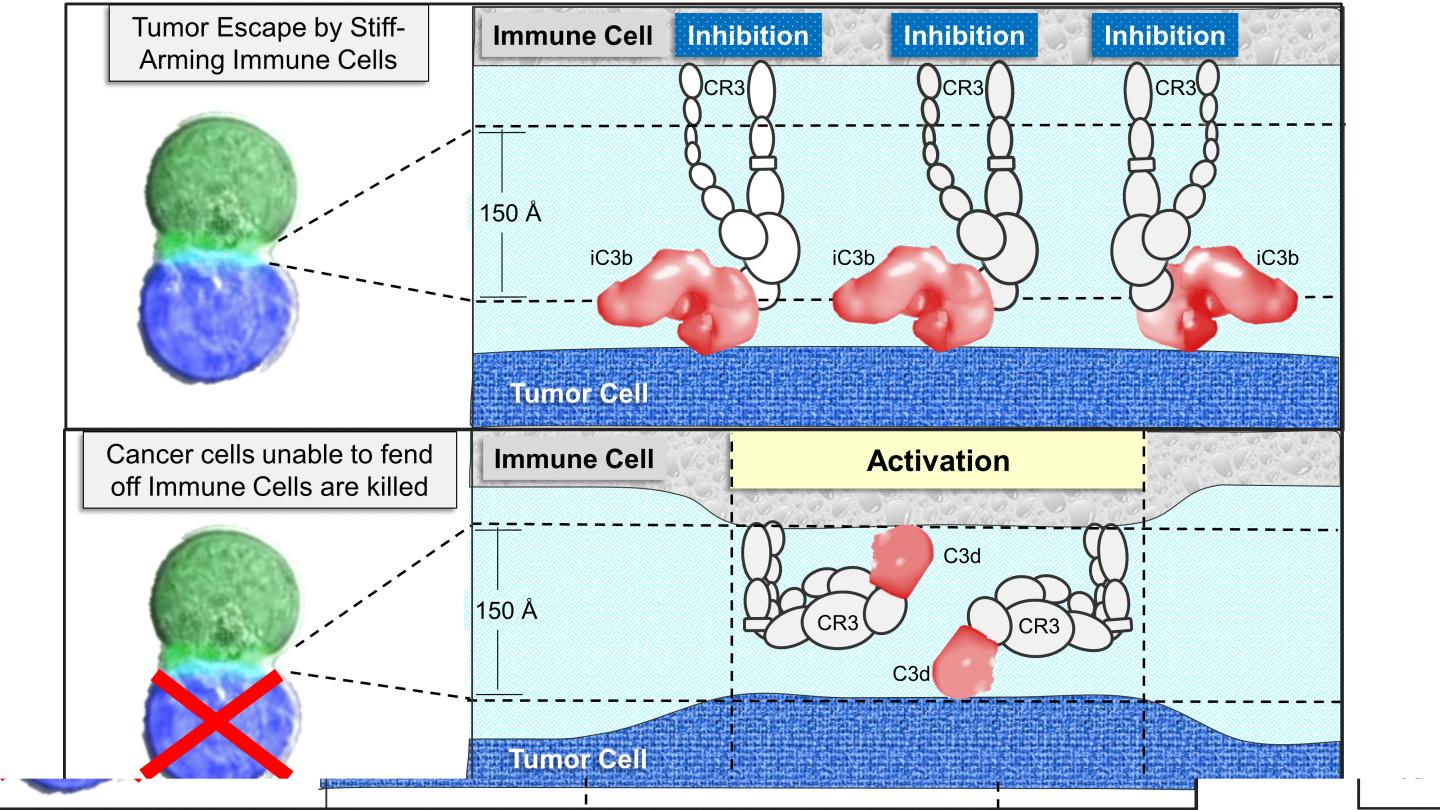
The “I belong” tag (called iC3b in the paper) is much larger in size that the “I don’t belong” tag (called C3d in the paper) that identifies unwanted cells. Both tags are made from the same complement protein (called Complement C3 in the paper). Host cells are able to process C3 to produce the “I belong” iC3b tag by inhibiting production of the “don’t belong” C3d tag. They have a set of proteins on their surface designed to prevent C3d tag formation. Bacteria and other foreign cells lack these proteins and end up covered with the C3d tag, leading to their destruction by immune cells. Surprisingly, the receptor (called CR3 in the paper) for the “I belong” and is the same as the one for the “I don’t belong” tags. How then does the immune system know which tag is which? This is the question Dr. Herbert addresses in the paper.
It turns out that the CR3 receptor can change conformation just like an arm can be either flexed or extended. The receptor CR3 binds the “I belong” tag most strongly when it is extended. It prevents immune cells from attacking by pushing them away, the molecular equivalent to a stiff-arm. In contrast, the receptor binds the “don’t belong” tag when it is flexed, drawing the immune cells close enough to engage. The defenders can then check for abnormal proteins. If they find any that “don’t belong,” they then respond to eliminate the threat. To avoid facing sudden death, tumors overproduce the “I belong” tag so that they appear normal rather than dangerous.
Cancer cells have many different strategies for coating their surface with “I belong” tags. These tags enable the tumors to escape attack, preventing an anti-tumor immune response from developing and long-term immunity from forming. Tumors can overproduce all the components necessary to load their surface with the “I belong” tag. Alternatively, they can capture the “I belong” tag from their immediate surroundings. In one example, tumors take advantage of bacteria that grow inside them. The bacteria initiate complement activation. The tumors then need produce only those proteins necessary to coat their surface with the “I belong” iC3b tag. In return, the lack of immune response also enables the bacteria to survive.
The complement system was first characterized by its ability to work with antibodies to eliminate disease-causing bacteria. Many talented scientists have worked over the last 130 years to characterize its many components with many features now revealed at atomic resolution. This work led to an understanding of how gene mutations that impair the “I belong” tagging process cause inflammatory diseases like glomerulonephritis and auto-immune hemolytic anemias, as well as the development of new therapeutics to treat these disorders. The work in the current paper builds on these findings to explain how this ancient system for self-nonself discrimination is exploited by tumors to ensure their survival. While the new insights are surprising after such a long and storied history, it does suggest that on occasions, an old dog may still have some new tricks to show you.
InsideOutBio is an early-stage biotech company developing therapeutics for the treatment of cancer based on the “I don’t belong” tag. By correctly identifying cancer cells as abnormal, the therapeutics initiate responses against them, leading to their rejection. Proof of principle has been accomplished in pre-clinical models. The company is operating remotely and has taken advantage of the new biotech ecosystem to discover and prototype the new therapeutics at low cost. The access to the enormous databases created by collaborative international efforts has helped the InsideOutBio scientists make fundamental discoveries such as reported by Dr. Herbert in the publication. Previously Dr. Herbert discovered a biological role for the left-handed Z-DNA conformation, recently confirmed by multiple labs after years of controversy and performed pioneering human genetic studies in the Framingham Heart Study. InsideOutBio is privately funded .
###
Media Contact
Alan Herbert
[email protected]
Related Journal Article
http://dx.