Pancreatic cancer remains a formidable adversary in the realm of oncology, characterized by its stealthy progression and dire prognosis. Among its variants, pancreatic ductal adenocarcinoma (PDAC) is particularly notorious, accounting for approximately 90% of all pancreatic cancer cases. Early detection is rare, largely due to the subtlety of initial symptoms, which often leads to a diagnosis at an advanced stage. Unfortunately, this late detection significantly limits the efficacy of surgical interventions, resulting in a staggering 70% of PDAC patients experiencing lymph node metastasis at the time of diagnosis. This underscores the urgent need for advanced diagnostic and therapeutic strategies tailored to this malignancy.
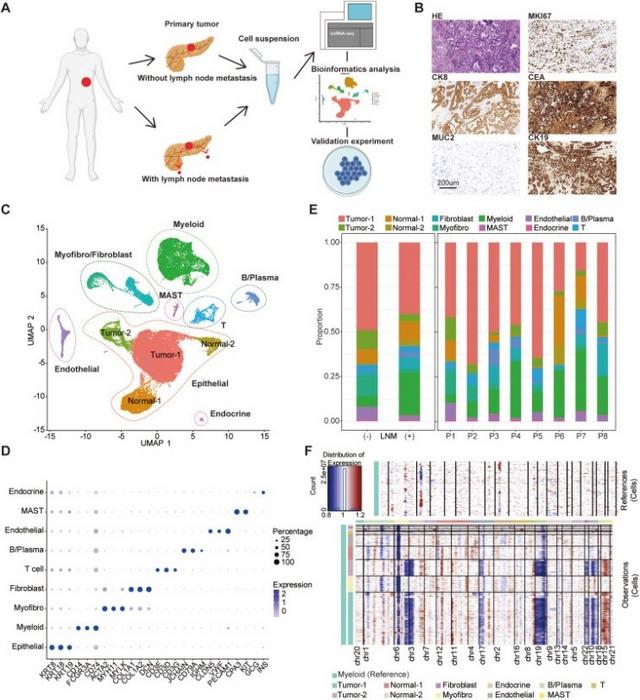
A recent landmark study published in the esteemed journal Genes & Diseases has embarked on a path to unravel the intricate genetic architecture of PDAC through the innovative lens of single-cell RNA sequencing (scRNA-seq). Conducted by a team of adept researchers at Fudan University Shanghai Cancer Center in conjunction with Shanghai Jiao Tong University School of Medicine, the study focuses on eight PDAC patients with varying statuses of lymph node metastasis. By scrutinizing the transcriptional profiles of individual cells, the researchers aim to elucidate the cellular dynamics that govern PDAC progression and cancer metastasis, endeavoring to identify potential therapeutic targets that could revolutionize treatment paradigms.
The scRNA-seq analysis revealed a striking heterogeneity within the cellular composition of PDAC samples, leading to the identification of four unique cell populations that are instrumental in tumor biology. Among these cell types are the MMP1+ and S100A2+ tumor cells, CCL2+ macrophages, and OMD+ fibroblasts. This multifaceted approach offers profound insights into the tumor microenvironment, highlighting the dynamic interplay among cancer cells, immune system components, and stromal cells. As the understanding of these relationships deepens, it presents opportunities for targeted interventions that could disrupt the pro-tumor signaling pathways crucial for PDAC progression.
The integrated analysis comparing PDAC tissues with normal pancreatic epithelial cells has unearthed a specific subset of pancreatic intraepithelial neoplasia (PanIN) with elevated expression of ONECUT2, a gene implicated in cellular differentiation. The findings suggest that this subset may act as a precursor to PDAC, displaying heightened levels of MMP1, a matrix metalloproteinase linked to poor prognosis in pancreatic cancer. The upregulation of MMP1 is particularly noteworthy as it plays a pivotal role in maintaining ductal identity during the transition from normal acinar cells to malignant tumor cells.
Delving further into the S100A2+ subset, researchers discovered increased expression of genes associated with tumor progression. Experimental studies conducted in vitro revealed that S100A2 significantly enhances the migratory capacity of cancer cells, establishing a connection between its elevated expression and aggressive tumor behavior. Interestingly, the knockdown of S100A2 resulted in a marked decrease in epithelial-mesenchymal transition (EMT) gene expression, underscoring its importance in the pathways driving metastasis and chemoresistance.
A thorough examination of the immune cell landscape within the PDAC microenvironment revealed an alarming presence of pro-metastatic immune cell subsets in cases of lymph node metastasis, significantly more than in non-metastatic PDAC tumors. This pro-metastatic milieu was predominantly characterized by a high abundance of CCL2+ macrophages, which have been implicated in the mechanisms of immune evasion and tumor progression. These macrophages are not only involved in promoting EMT and immunosuppression but also in facilitating the indirect activation of cytotoxic CD8+ T cells, further complicating the immune response against the tumor.
In parallel, the analysis of stromal cells identified a distinct population of OMD+ fibroblasts that are crucial in fostering a pro-tumor environment. These fibroblasts play a dual role: they not only regulate tumorigenesis but also facilitate metastasis by recruiting CCL2+ macrophages, thereby contributing to a nurturing environment that promotes PDAC progression. This intricate interplay between fibroblasts and macrophages emphasizes the necessity for a comprehensive understanding of the tumor microenvironment in order to devise effective therapeutic modalities.
This pioneering study offers significant insights, suggesting that targeting these identified cell populations—OMD+ fibroblasts, CCL2+ macrophages, S100A2+, and MMP1+ tumor cells—could open new avenues for therapeutic interventions in PDAC. By focusing on these key players within the tumor ecosystem, there is potential for developing targeted therapies aimed at disrupting the communication networks that fuel cancer growth and metastasis. Such strategies could not only improve patient outcomes but also shift the paradigm in how pancreatic cancer is treated.
The novel findings highlighted in this research underscore the critical need for ongoing investigations into the cellular and molecular intricacies of PDAC. By unraveling the genetic and cellular frameworks that underpin tumor biology, researchers can pave the way for the identification of biomarkers that predict treatment responses and disease progression. Furthermore, integrating this knowledge with technological advancements in personalized medicine could enhance therapeutic efficacy and patient survival rates.
As we navigate an era marked by rapid advancements in genomic technologies and precision medicine, it is imperative to leverage these insights into actionable clinical strategies. The implications of this study extend beyond academic curiosity; they hold the promise of transforming how we understand, detect, and treat pancreatic cancer. By synthesizing research findings into clinical practice, there is hope that the tide can be turned in favor of patients battling this formidable disease.
The urgent need for collaborative efforts among researchers, clinicians, and policymakers cannot be overstated. The battle against pancreatic cancer demands a multifaceted approach that not only focuses on individual treatments but also addresses the broader landscape of cancer biology and patient care. By fostering interdisciplinary collaborations and encouraging innovation within research settings, we can aspire to bring about meaningful changes in the fight against pancreatic cancer.
To conclude, this pioneering study shines a much-needed light on the complexities of PDAC, revealing potential paths forward in the development of targeted therapies. It stands as a testament to the resilience and ingenuity of the scientific community in confronting one of the most challenging cancers known to humanity. As we continue to unravel the mysteries of cancer biology, the hope of improving outcomes for patients with pancreatic cancer becomes not just an aspiration, but a tangible goal within our reach.
Subject of Research: The transcriptional landscape of pancreatic ductal adenocarcinoma and its cellular composition.
Article Title: The scRNA-sequencing landscape of pancreatic ductal adenocarcinoma revealed distinct cell populations associated with tumor initiation and progression.
News Publication Date: October 2023.
Web References: Genes & Diseases Journal
References: DOI: 10.1016/j.gendis.2024.101323
Image Credits: Credit: Genes & Diseases
Keywords: Pancreatic cancer, pancreatic ductal adenocarcinoma, single-cell RNA sequencing, tumor microenvironment, lymph node metastasis, MMP1, S100A2, CCL2, fibroblasts, macrophages.
Tags: advanced diagnostic strategies for PDACcellular dynamics in cancer progressionearly detection of pancreatic cancerFudan University pancreatic cancer studygenetic architecture of pancreatic tumorslymph node metastasis in PDAConcology research breakthroughspancreatic ductal adenocarcinoma researchPDAC patient outcomes and prognosissingle-cell RNA sequencing in cancertherapeutic strategies for pancreatic cancertranscriptional landscape of pancreatic cancer