Researchers from the National Institute for Physiological Sciences in Japan successfully grow mouse stem cells into sperm in the body of the rat
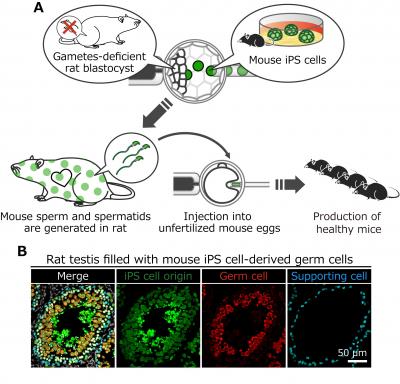
A. Schematics for the generation of rats with mouse gametes. Mouse spermatids isolated from the rat testis are injected into unfertilized mouse eggs to produce viable mice.
Okazaki, Japan – Making gametes such as sperm and eggs from pluripotent stem cells, primitive cells that can make all the tissues, greatly contributes to efficient reproduction of livestock animals and future assisted reproductive medicine. Researchers pave the way to achieve this goal using a body of xenogenic animals.
The researchers previously developed a method to grow stem cells into an entire organ in the body, so-called blastocyst complementation. The blastocyst is a structure of early embryos. If stem cells are transplanted into the blastocyst obtained from animals that cannot make a certain organ, the stem cells compensate the missing organ in the developing body, and make the entire organ. “We expected this method is also applicable to the efficient production of gametes,” explains an author who led the study, Dr. Toshihiro Kobayashi.
A year ago, the researchers created a genetically modified rat that completely lacks sperm and eggs. They hypothesized that the rat can be used as an excellent host to grow exogenous stem cells into gametes.
The researchers firstly transplanted allogenic rat stem cells into blastocyst obtained from rats that are unable to make gametes, and confirmed all the gametes were derived from the stem cells. The generated gametes deliver the genetic information from the stem cells to the next generation, which enables efficient production of genetically modified rats.
Then, researchers tested whether xenogenic mouse stem cells can make mouse gametes in the body of rats. Remarkably, mouse germ cells including sperm and spermatids were observed in the testis of the rats, and the spermatids could fertilize with mouse eggs to produce healthy pups (see figures).
“Making gametes from stem cells even in the xenogenic environment is quite important for the application of this strategy,” says another author led the study, Dr. Masumi Hirabayashi. “In the future, we may be able to use the method to preserve endangered species, since their stem cells are available due to iPS cell technology.”
###
Media Contact
Toshihiro Kobayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




