CAMBRIDGE, MA — MIT chemists have devised a new chemical reaction that allows them to synthesize a phosphorus-containing ring, using a catalyst to add phosphorus to simple organic compounds called alkenes.
Credit: MIT
CAMBRIDGE, MA — MIT chemists have devised a new chemical reaction that allows them to synthesize a phosphorus-containing ring, using a catalyst to add phosphorus to simple organic compounds called alkenes.
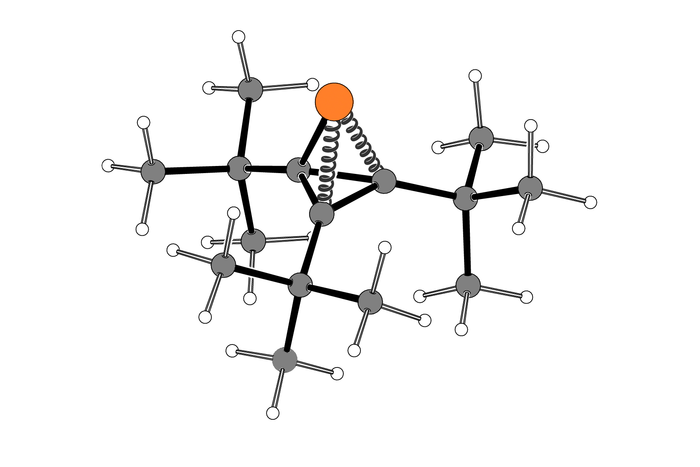
Their reaction, which yields a ring containing two carbon atoms and one phosphorus atom, can be performed at normal temperature and pressure, and makes use of a novel “spring-loaded” phosphorus-containing molecule that supplies the phosphorus atom.
“This is a rare example of a discovery of a new catalytic reaction, and it opens up a real wealth of new opportunities to do chemistry enabled by a reaction that never existed before,” says Christopher Cummins, the Henry Dreyfus Professor of Chemistry at MIT and the senior author of the study.
These phosphorus-containing rings could find uses as catalysts for other reactions, or as precursors for useful compounds such as pharmaceuticals, Cummins says.
MIT graduate student Martin-Louis Riu is the lead author of the paper, published this week in the Journal of the American Chemical Society. Former MIT research fellow Andre Eckhardt is also an author of the study.
Creating a ring
Organic compounds that contain double bonds between carbon atoms, also known as olefins or alkenes, are important precursors in many industrially useful chemical reactions. By breaking those carbon-carbon bonds and adding new atoms or groups of atoms, researchers can create a wide variety of new products.
As one example, chemists have previously devised ways to convert a carbon-carbon double bond into a three-membered ring by adding either another carbon atom, a nitrogen atom, or an oxygen atom. Such compounds can be found in plastics, pharmaceuticals, textiles, and other useful products.
However, because phosphorus is heavier than carbon, nitrogen, or oxygen, it has been difficult to find a way to incorporate it into olefins without using “brute force” methods that require harsh chemical conditions. The MIT team wanted to come up with a way to perform this reaction under mild conditions, using a catalyst to transfer a phosphinidene group — a phosphorus atom bound to an organic chemical group — to the olefin.
In order to achieve that, they needed a starting material that could act as a source of phosphinidene, but such compounds did not exist because direct analogues of those used for lighter elements such as carbon are unstable with phosphorus.
In a 2019 paper, Cummins’s lab developed one possible source, consisting of phosphinidene attached to a molecule that contains several hydrocarbon rings. Using this compound, they were able to synthesize a three-membered ring containing phosphorus, but the reaction required high temperatures and only worked with certain types of olefins.
In their new paper, the MIT team used a different source of phosphorus for the reaction — a compound that Cummins’ lab first synthesized in 2021. This molecule is a tetrahedron, a shape that inherently has a great deal of energy “strain,” much like a compressed spring, because of the small bond angles between the four atoms that form the tetrahedron.
This compound, called tri-tert-butylphosphatetrahedrane, has three vertices consisting of carbon atoms attached to a chemical group called tert-butyl, and one vertex consisting of a phosphorus atom with an unshared pair of electons. Under the right conditions, this strained molecule can be broken apart to release the phosphorus atom.
Efficient synthesis
Using this spring-loaded molecule, the researchers were able to use a nickel-containing catalyst to transfer phosphinidene to olefins to create three-membered rings. This reaction can be done at room temperature, with high yield of the desired product.
“All the stars aligned here in terms of us being able to synthesize a highly strained precursor that leads to room temperature reactivity and rapid catalysis,” Cummins says.
The researchers now plan to further investigate the mechanism of how this reaction occurs, which they believe is dependent on phosphinidene being temporarily transferred to the nickel catalyst complex. The catalyst then incorporates the phosphorus into the double bond of the olefin.
They also hope to explore the possibility of creating a variety of new compounds that include the phosphorus-containing ring, and to develop ways to control which of two possible mirror image versions are synthesized. Once these phosphorus-containing rings are formed, they can be opened up by adding additional molecules to create other useful compounds. Potential applications for these kinds of products include catalysts for other reactions, or components of pharmaceuticals that contain phosphorus.
###
The research was funded by the National Science Foundation and a Feodor Lynen Research Fellowship from the Alexander von Humboldt Foundation.
Journal
Journal of the American Chemical Society
DOI
10.1021/jacs.2c02236
Article Title
Reactions of tri-tert-butylphosphatetrahedrane as a spring-loaded phosphinidene synthon featuring nickel-catalyzed transfer to unactivated alkenes
Article Publication Date
19-Apr-2022




