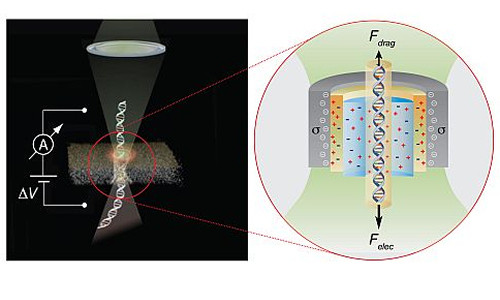
The Opto-electrical effect can be used to control the passage of DNA molecules through nanopore sensors, thereby leading to more accurate sensing and sequencing of individual DNA molecules
Low-cost, ultra-fast DNA sequencing would revolutionize healthcare and biomedical research, sparking major advances in drug development, preventative medicine and personalized medicine. By gaining access to the entire sequence of your genome, a physician could determine the probability that you’ll develop a specific genetic disease or tolerate selected medications. In pursuit of that goal, Professor Amit Meller (BME) has spent much of the past decade spearheading a method that uses solid state nanopores: 2 – 5 nanometer-wide holes in silicon chips that read DNA strands as they pass through to optically sequence DNA molecules.
Now Meller and a team of researchers at the Technion and Boston University have discovered a simple way to improve the sensitivity, accuracy and speed of the method, making it an even more viable option for DNA sequencing or characterization of small proteins, such as Ubiquitin in their native, folded, state.
In the November 3 online edition of Nature Nanotechnology, the team demonstrated that focusing a low-power, commercially available green laser on a nanopore increases current near walls of the pore, which is immersed in salt water. As the current increases, it sweeps the salt water along with it in the opposite direction of incoming samples. The onrushing water, in turn, acts as a brake, slowing down the passage of DNA through the pore. As a result, the nanoscale sensors can get a higher-resolution read of the DNA as it crosses the pore, and identify small, proteins that could not previously be detected.
Meller: “The light-induced surface charge modulation phenomenon that we describe in this paper can be used to instantly switch on and off the “brakes” acting on individual biopolymers, such as DNA or proteins sliding through the nanopores. This critically enhances the sensing resolution of solid-state nanopores, and can be easily integrated in future nanopore based DNA sequencing and protein detection technologies”
Slowing down DNA is essential to DNA or RNA sequencing with nanopores, so that nanoscale sensors can make the right call on what’s passing through.
“The goal is to hold a base pair (of DNA nucleotides) in the nanopore’s sensing volume long enough to ‘call the base’ (i.e, determine if it’s an A, C, G or T),” said co-author Allison Squires (a Boston University student, who also worked at the Technion and fabricated nanopores in the study). “The signal needs to be sufficiently different for each base for sensors in the nanopore to make the call. If the sample proceeds through the sensing volume too quickly, it’s hard for the sensors to interpret the signal and make the right call.”
Meller and his team characterized the amount of increase in current under varying illumination in many different-sized nanopores. They next aim to explore in greater detail the mechanism underlying the increase in surface current when the green laser is applied to a nanopore, information that could lead to even more sensitivity and accuracy in DNA sequencing.
Story Source:
The above story is reprinted from materials provided by Israel Institute of Technology.