Researchers from Osaka University report a straightforward approach to protein modification, providing a new tool for protein engineering
Credit: Osaka University
Osaka, Japan – Proteins are essential parts of organisms; thus, they are widely used in medicine, biology and chemistry. Enhancing their inherent properties by adding functional molecules to their structures is a common and important step in many fields. For example, adding fluorescent molecules allows proteins to be traced and quantified. Many different modification strategies with various advantages have been described. Osaka University researchers now report a simple N terminus-specific modification carried out under mild conditions using new reagents prepared in one step. Their findings were published online in ChemBioChem.
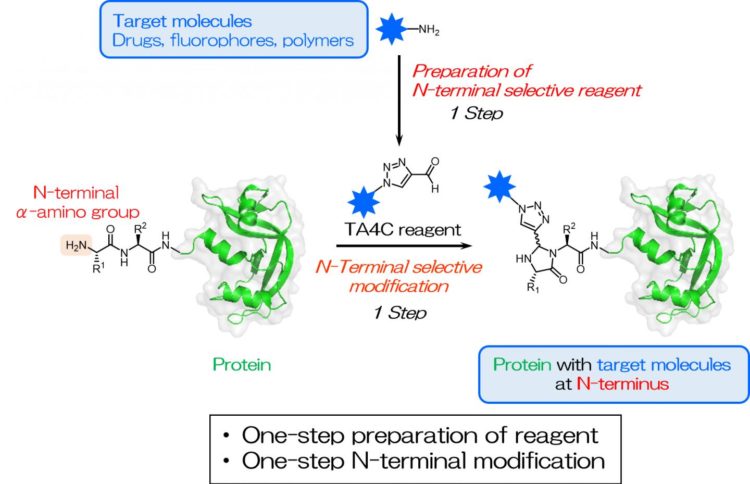
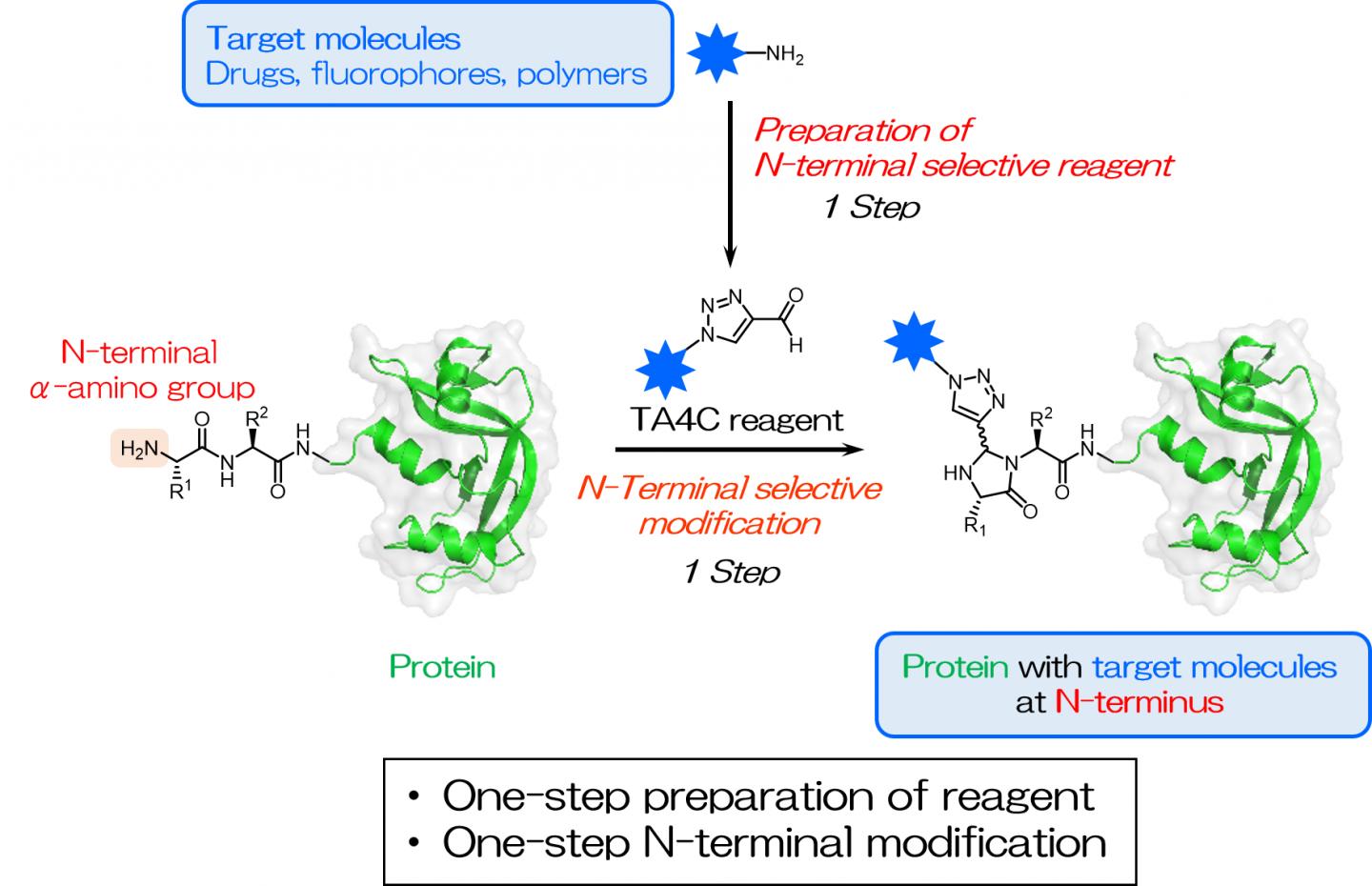
The N terminus is defined as the beginning of the protein chain where the amino group of the first amino acid building block is available to react. Specifically targeting the N terminus is useful as it is rarely involved in the protein folding, making it easily accessible while having minimal impact on the protein function. It is known to be a unique and ever-present site within each protein.
Inspired by previous works, the researchers screened a series of cyclic nitrogen-containing compounds and found that 1H-1,2,3-triazole-4-carbaldehyde (TA4C) derivatives can be conjugated to the N-terminus in a single step with relatively high conversions, up to 92%.
“Simplifying protein modification is a valuable development for a variety of fields,” corresponding author Akira Onoda explains. “Our approach results in highly efficient site-specific labeling under mild conditions, which is important when working with sensitive biological molecules. As long as the molecule to be added contains an amino group, a reaction can be carried out to create the TA4C group in one step, which is then reactive towards the protein N terminus.”
The TA4C reagents are prepared in a single step from a functional molecule with an amino group via a reaction known as the Dimroth rearrangement. A variety of amine-containing molecules were successfully used, including polyethylene glycol, biotin, and fluorescein, demonstrating the wide range of possible functionalities.
“We believe our approach will contribute as an immensely practical option to the protein modification toolbox and accelerate development in many areas which rely on protein conjugation,” corresponding author Takashi Hayashi explains. “In addition, combining our approach with techniques that target other protein sites will allow multiple functions to be introduced, providing great flexibility. This will prove beneficial in a wide variety of fields including bioengineering, pharmaceuticals, and diagnostics.”
###
The article, “Triazolecarbaldehyde reagents for one-step N-terminal protein modification” was published in ChemBioChem at DOI: https:/
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
Original Source
https:/
Related Journal Article
http://dx.