Chagas disease has long been recognized as a silent and insidious health threat across the Americas, often escaping detection until it manifests in severe, life-threatening complications. This neglected tropical disease is caused by the protozoan parasite Trypanosoma cruzi, which is transmitted through the feces of infected triatomine bugs, colloquially known as kissing bugs. Named for their biting preference around the mouth and face during the night, these insects inadvertently spread the parasite to millions of people, many of whom remain unaware of their infection for decades. Researchers at the University of Cincinnati are now advancing our understanding of this elusive pathogen with the hope of unveiling new therapeutic interventions.
At the heart of this research effort is Noelia Lander, an assistant professor whose molecular parasitology laboratory is dedicated to unraveling the complexities of T. cruzi’s lifecycle and identifying its vulnerabilities. The parasite’s remarkable ability to adapt to wildly different environments—from the midgut of the insect vector to the bloodstream and tissues of mammalian hosts—poses a formidable challenge. Over millions of years of evolution, T. cruzi has developed intricate mechanisms to survive drastic changes in pH, temperature, and nutrient availability. Dissecting these adaptations at a molecular level could provide critical targets to disrupt the parasite’s progression and ultimately halt the disease.
Trypanosoma cruzi undergoes a highly dynamic lifecycle involving multiple distinct forms, each tailored to different stages of infection and survival. Once introduced to a human host through the bite wound contaminated by kissing bug feces, the parasite invades various cell types, establishing chronic infection. This intracellular lifestyle poses a significant obstacle to the immune system and pharmacological treatments, allowing the parasite to evade immune detection and persist silently for years. Compounding the problem, existing drugs tend to lose efficacy in the chronic phase, underscoring the urgent need for novel strategies that intervene earlier or target these hidden reservoirs.
.adsslot_Y12lOTH9zS{ width:728px !important; height:90px !important; }
@media (max-width:1199px) { .adsslot_Y12lOTH9zS{ width:468px !important; height:60px !important; } }
@media (max-width:767px) { .adsslot_Y12lOTH9zS{ width:320px !important; height:50px !important; } }
ADVERTISEMENT
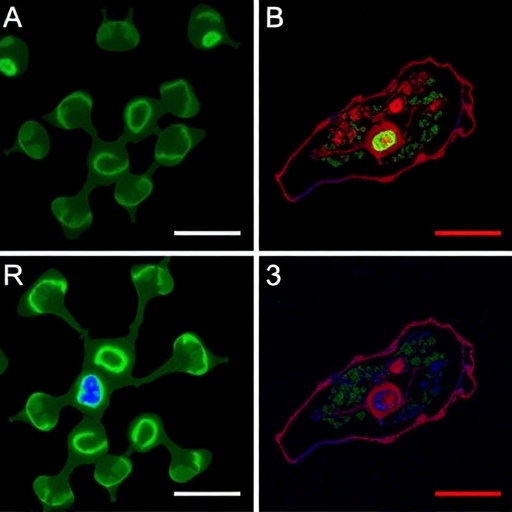
Using cutting-edge gene editing technologies, Lander and her colleagues have begun probing the genomic and proteomic machinery that facilitates T. cruzi’s adaptability and infectivity. A recent study led by graduate student Joshua Carlson, alongside co-author Milad Ahmed, explored the function of a unique set of proteins believed to mediate cellular signaling processes crucial for the parasite’s survival. Among these, a protein called TcCARP3 has emerged as a pivotal modulator of compartmentalized cyclic AMP (cAMP) signals, which regulate crucial physiological responses such as osmoregulation—a mechanism by which the parasite controls its internal water balance in response to osmotic stress.
This modulation by TcCARP3 influences not only the parasite’s ability to endure hostile environments but also its efficiency in infecting mammalian cells and colonizing the triatomine vector. These findings suggest that TcCARP3 acts as a central hub that enables T. cruzi to coordinate its complex lifecycle transitions and environmental responses. Targeting such a molecular linchpin with therapeutic agents could prove transformative, potentially incapacitating the parasite by preventing it from adjusting to the diverse challenges it encounters during infection.
Although Chagas disease has traditionally received less attention than other vector-borne diseases, its epidemiological footprint is staggering. It is estimated that upwards of 6 to 8 million people across the Americas carry chronic infections, with approximately 300,000 cases documented in the United States alone. The insidious nature of this disease lies in its latency: individuals may live asymptomatically for decades before cardiac, digestive, or neurological complications culminate in life-threatening pathology. As UC Assistant Professor Lander explains, “The main issue with Chagas disease as a public health problem is that most people don’t know they’re infected until symptoms appear and it’s too late to treat them.”
This silent progression underscores the necessity of molecular studies that seek to identify weaknesses in the parasite’s lifecycle that can be exploited before irreversible organ damage occurs. The multifunctional role of cAMP signaling and TcCARP3 in enabling T. cruzi’s durability and infectivity positions these molecular pathways as attractive candidates for drug development. Interfering with the parasite’s signaling networks could shut down its ability to undergo key lifecycle transitions, effectively halting its propagation within human hosts and insect vectors alike.
Moreover, the evolutionarily ancient nature of T. cruzi, having existed long before humans emerged, contributes to its robustness and adaptability. It has exquisitely tuned its biology to survive extreme environmental shifts during transmission between hosts, a fact that both fascinates and challenges researchers. Dr. Lander articulates a nuanced perspective on this parasite: “I know the parasite is the enemy. But I’m impressed by the mechanisms the parasite has to survive during its lifecycle. The goal is to find its weaknesses to fight the disease.” This scientific admiration fuels meticulous inquiries into the parasite’s biochemistry and cell biology.
The collaborative efforts within Lander’s lab integrate multiple disciplines, ranging from molecular genetics and cell biology to vector ecology and immunology. Graduate students and postdoctoral researchers contribute to a comprehensive understanding of parasite physiology, using advanced microscopy, molecular assays, and computational modeling. This integrative methodology brings the research closer to identifying targeted interventions that bypass the current limitations of Chagas treatments, which often come with significant toxicity and variable efficacy.
Ongoing work aims to delineate further adaptations of T. cruzi at the subcellular level, exploring how compartmentalized signaling regulates not only osmoregulation but also metabolic fluxes, immune evasion mechanisms, and replication dynamics. Understanding these processes in fine detail could unveil novel drug targets or vaccine candidates. Interrupting the parasite’s capacity to transform between lifecycle stages might render it unable to complete infection or persist in host tissues, offering a strategic point of attack.
In summary, the research emerging from the University of Cincinnati provides a promising vision for combating Chagas disease by unveiling critical components of Trypanosoma cruzi’s adaptive machinery. The identification of TcCARP3 as a central orchestrator of cAMP-mediated signaling in parasite osmoregulation and mammalian infection marks an important advance in the field. These discoveries offer hope for the development of next-generation therapeutics designed to interrupt the parasite’s lifecycle, preventing disease progression and improving outcomes for millions of people affected worldwide.
Subject of Research: Cells
Article Title: TcCARP3 modulates compartmentalized cAMP signals involved in osmoregulation, infection of mammalian cells, and colonization of the triatomine vector in the human pathogen Trypanosoma cruzi
News Publication Date: 23-May-2025
Web References: https://journals.asm.org/doi/full/10.1128/mbio.00994-25
Image Credits: Andrew Higley
Keywords: Parasitology, Epidemiology, Human health
Tags: Chagas disease researchevolutionary adaptations of parasiteshost-parasite interactionskissing bug transmissionlifecycle of Trypanosoma cruzimolecular parasitology studiesneglected tropical diseasespublic health challenges in the Americastherapeutic interventions for Chagasunderstanding parasitic infectionsUniversity of Cincinnati research initiativesvector-borne diseases