Credit: MSU
EAST LANSING, Mich. – Depression affects women nearly twice as much as men, but unraveling the brain’s blueprint that regulates this behavior, let alone identifying specific molecular differences between sexes, has proven difficult.
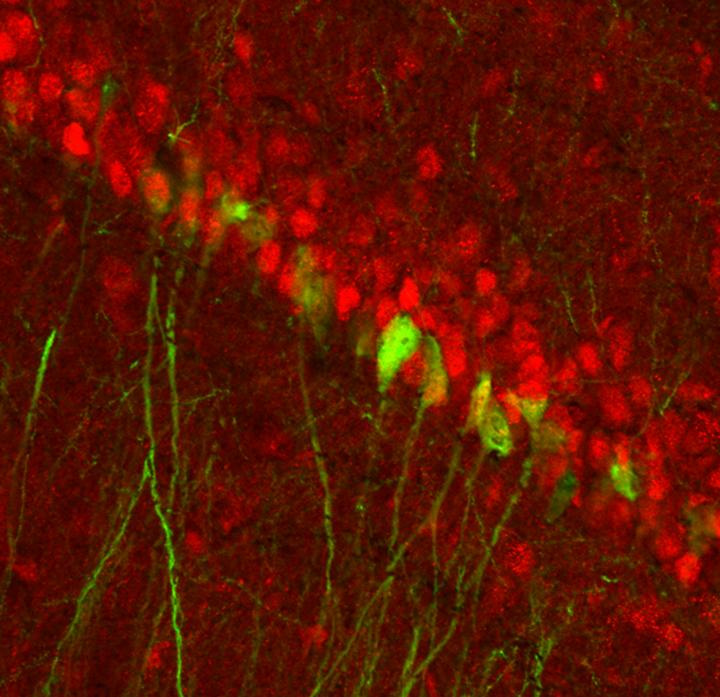
Michigan State University researchers, however, have found and flipped a switch in the brain, revealing a single circuit in mice that activates during stress and is controlled by testosterone. The results, published in Biological Psychiatry, focus on the activity between neurons in the ventral hippocampus, which become active under stress and emotion, and their activation of nucleus accumbens neurons, critical players in reward and motivation.
“What makes these findings stand out is not only identifying this new circuit,” said A.J. Robison, MSU physiologist and lead author of the study, “but also observing and confirming how it drives different behaviors in males and females.”
Oddly enough, many circuit-specific animal model studies involving depression-related behaviors don’t include female subjects. This gap exists despite sex differences in several depression-related brain regions, including the hippocampus, Robison added.
To help close this void, Robison and a team of MSU scientists focused on this hippocampus-accumbens circuit and saw that the activity in male brains during stress was significantly lower than in females, and this required testosterone. When they removed testosterone, however, the male mice began expressing depression-like behaviors.
Conversely, the team observed increased circuit activity in female brains, but when testosterone was introduced, the neurons quieted, and the female mice became resistant to the depression-like behaviors.
“Even with our best antidepressants, such as Prozac, we don’t know exactly how they work,” Robison said. “This is the first time we’ve found a circuit that drives this sexually different behavior; other scientists can now explore how this could translate to identifying new therapeutic targets in humans.”
Robison’s group used chemogenetic tools to manipulate specific circuit activity in the mouse brain in this study. Such tools may inform the development of “genetic medicine” for the treatment of human diseases in the future.
###
Additional MSU scientists who contributed to this research include: Elizabeth Williams, Claire Manning, Andrew Eagle, Ashlyn Swift-Gallant (now at Memorial University of Newfoundland), Natalia Duque-Wilckens, Sadhana Chinnusamy, Adam Moeser, Cynthia Jordan and Gina Leinninger.
This research was funded in part by the National Institutes of Mental Health, the National Institutes of Neurological Disease and Stroke, the National Institutes of Drug Abuse and the Avielle Foundation.
(Note for media: Please include a link to the original paper in online coverage: https://www.biologicalpsychiatryjournal.com/article/S0006-3223(19)31618-X/pdf)
Michigan State University has been working to advance the common good in uncommon ways for 160 years. One of the top research universities in the world, MSU focuses its vast resources on creating solutions to some of the world’s most pressing challenges, while providing life-changing opportunities to a diverse and inclusive academic community through more than 200 programs of study in 17 degree-granting colleges.
For MSU news on the Web, go to MSUToday. Follow MSU News on Twitter at twitter.com/MSUnews.
Media Contact
Layne Cameron
[email protected]
517-353-8819
Original Source
http://go.
Related Journal Article
http://dx.




