Mice engineered for SARS-1 studies repurposed for novel coronavirus
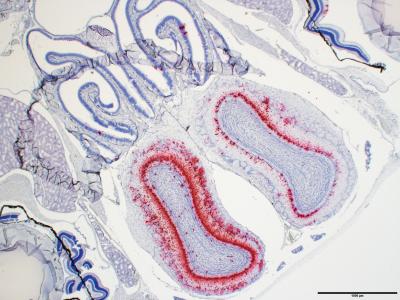
Credit: Joseph W. Golden and Xiankun Zeng, USAMRIID
Army scientists have developed the first lethal mouse model of SARS-CoV-2, the virus that causes COVID-19, using mice that were genetically engineered to express the human ACE2 gene–a key mechanism by which the virus enters human cells. In addition to shedding light on the pathogenesis of COVID-19, this work directly contributes to the advancement of medical countermeasures against the virus.
A preview of the paper, authored by Joseph W. Golden, Ph.D. and colleagues at the U.S. Army Medical Research Institute of Infectious Diseases, appears online this week in JCI Insight, a journal published by the American Society for Clinical Investigation.
Developing a small animal model that mirrors the course of human disease is an essential step in advancing vaccines, diagnostics, and treatments to combat this international health crisis. Previous research on SARS-CoV, the virus responsible for the 2003 global outbreak of Severe Acute Respiratory Syndrome, revealed that the virus binds to target cells by means of an interaction between the 139 kDa viral spike protein and the host angiotensin-converting enzyme 2, or ACE2, protein.
The novel coronavirus, SARS-CoV-2, uses the same mechanism to infect cells. In normal mice, however, the virus does not easily bind to ACE2, making it difficult to study the course of infection. So Golden and the USAMRIID team used a special type of mice called K18-hACE2, which are specially bred with the human ACE protein instead of the mouse version. This mouse strain, developed for the original SARS-CoV outbreak by the Stanley Perlman laboratory at the University of Iowa in the early 2000s, was revived and recently produced at the Jackson Laboratory.
USAMRIID’s study used two groups of 14 K18-hACE2 mice, infected with two different doses of SARS-CoV-2 administered by the intranasal route, to simulate how humans are exposed to the virus. Each group contained seven male and seven female mice. On day 3, four mice per group were euthanized to assess disease severity, while the remaining ten mice per group were monitored up to 28 days. Overall, the K18-hACE2 mice developed acute disease, including weight loss, lung injury, and brain infection, and ultimately succumbed to the disease, according to the authors.
In addition, the team infected three other types of mice–C57BL/6, BALB/c and RAG2 deficient mice–with a challenge dose of the virus. These animals did not lose weight and none succumbed to the disease.
“Given that the K18-hACE2 animal model is commercially available, it provides an important platform for evaluation of medical countermeasures across multiple laboratories,” said Golden.
###
About the U.S. Army Medical Research Institute of Infectious Diseases:
For over 50 years, USAMRIID has provided leading edge medical capabilities to deter and defend against current and emerging biological threat agents. The Institute is the only laboratory in the Department of Defense equipped to safely study highly hazardous viruses requiring maximum containment at Biosafety Level 4. Research conducted at USAMRIID leads to medical solutions – vaccines, drugs, diagnostics, information, and training programs – that benefit both military personnel and civilians. Established in 1969, the Institute plays a key role as the lead military medical research laboratory for the Defense Threat Reduction Agency’s Joint Science and Technology Office for Chemical and Biological Defense. USAMRIID is a subordinate laboratory of the U.S. Army Medical Research and Development Command. For more information, visit http://www.
Reference:
The in-press preview version of the article, “Human angiotensin-converting enzyme 2 transgenic mice infected with SARS-CoV-2 develop severe and fatal respiratory disease,” is available at this link: https:/
Authors:
Joseph W. Golden, Curtis R. Cline, Xiankun Zeng, Aura R. Garrison, Brian D. Carey, Eric M. Mucker, Lauren E. White, Joshua D. Shamblin, Rebecca L. Brocato, Jun Liu, April M. Babka, Hypaitia B. Rauch, Jeffrey M. Smith, Bradley S. Hollidge, Collin Fitzpatrick, Catherine V. Badger, and Jay W. Hooper, all of USAMRIID.
Funding:
Funding was provided through the Defense Health Program.
Media Contact
Caree Vander Linden
[email protected]




