When biology and materials science converge, the results can be new materials that can be used to deliver targeted drugs, repair damaged arteries or rebuild failing tissues, such as the anterior cruciate ligament, the ACL injury that can end sports careers.
Penn State bioengineer Jian Yang is developing polymers designed to target all three.
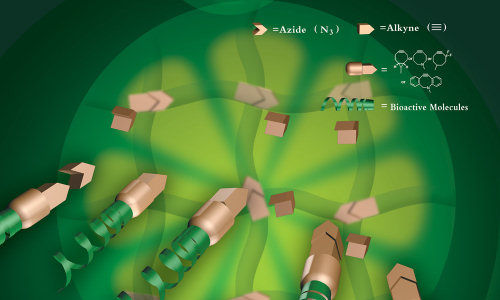
Tissue engineering with click chemistry
Finding the right balance between mechanical strength and elasticity in artificial tissue scaffolding has been problematic, as has been the need to add in the desirable traits of biocompatibility and controlled biodegradability. In a recent article in Advanced Materials, Yang and his colleagues in Penn State’s Department of Biomedical Engineering and the Academy of Orthopedics of Guangdong Province in China report on the use of thermal click chemistry to make crosslinked citrate-based biodegradable elastomers with high mechanical strength (up to 40 MPa of tensile stress) with easy surface biofunctionalization. In comparison, the ACL has a tensile strength of 38 MPa and most biodegradable elastomers have a dry tensile strength below 10MPa. Click chemistry is a relatively new technique used primarily in drug discovery that uses a few reliable reactions to lock or “click” together small units of biomaterials in simple processes. Yang believes that this is the first reported use of click chemistry to design versatile biodegradable elastomers for tissue engineering.
Beyond superior mechanical strength, Yang’s click polymers provide user-friendly and site-specific functionalization with bioactive molecules to, for instance, promote cell growth. In addition the click polymers show a desirable type of biodegradability he calls “first slow then fast.” In many tissue engineering applications the preservation of mechanical strength of the artificial scaffolding during the early period of tissue regeneration is important. Yet many elastomeric polymers begin to degrade at a steady rate after implantation. The click polymer in this study, called POC-click-3, had low degradation for a sustained period compared to other polymers, but then rapidly degraded.
Yang and colleagues believe that their clickable biodegradable elastomer design will greatly expand the application of biodegradable polymers in areas such as drug delivery, orthopedic fixation devices, tissue engineering and other types of medical implants.
Blood Vessel Repair
Yang has recently received funding from the National Institutes of Health on a project to develop nanoparticles that promote healing in damaged endothelium, the lining of blood vessels, which can be injured in surgical procedures that unblock clogged arteries.
“Angioplasty and stenting often damage arterial walls,” Yang explains, “with a significant risk of subsequent complications, such as re-narrowing of the artery or blood clot.” Platelets accumulate on the damaged vessel, initiating clot formation. Other cells can deposit on the cell wall, building up a blockage. The result is multiple surgeries and multiple stent replacements.
Yang and his co-PI Kytai Truong Nguyen at University of Texas Arlington will develop and test a polymer nanoparticle that mimics the platelet in the blood that forms the clot and create a cover over the damage. Their nanoparticle is decorated with a ligand called GP1b peptide that links to endothelial progenitor cells circulating in the blood that can grow into mature endothelial cells. Over time, the nanoparticles will degrade harmlessly as the new blood vessel lining repairs the damage, avoiding the need for a stent. “The surgeon will still do angioplasty first, but not put in a stent. Instead they will inject the nanoparticle solution, if necessary more than once,” Yang explains.
“Our nanoparticles have two functions: They will serve as a temporary template to cover injured vascular wall to prevent the underlying smooth muscle cells’ over growth inward to block the artery. Once the nanoparticles attach to the vessel wall the platelets cannot attach. Then the nanoparticles will catch the circulating endothelial progenitor cells to form a healthy endothelium on the injured vascular wall. Once the missions are done, the nanoparticles will simply disappear without causing any long-term toxicity. These injectable nanoparticles have worked well in animal models in studies with our collaborators at UT Arlington,” Yang says. The team received $1.4 million over four years from the National Institutes of Health to develop this technology.
Attacking prostate cancer
In a second recent award from NIH, Yang and co-principal investigator Jer-Tsong Hsieh, the Dr. John McConnell Distinguished Chair in Prostate Cancer Research at the University of Texas Southwestern Medical Center, received $1.6 million over five years to develop biodegradable nanoparticles to image and treat prostate cancer.
Prostate cancer is the second leading cause of cancer death in American men. If the cancer develops treatment resistance the tumours will keep growing and spread to other parts of the body. To prevent that, Yang and Hsieh will try to identify a prostate cancer specific drug called a genotoxin that will attack the cancer cells, and develop a fluorescent nanoparticle to target the cancer cells. Yang’s group plans to add magnetic resonance imaging particles to the fluorescent nanoparticles in order to find the exact location of the tumour. If surgical removal of the cancer is required, fluorescent nanoparticles will attach to cancer cells and help the surgeon identify those small clusters of cancer cells that are usually invisible to the eye. “We will need to optimize the genotoxin, and make sure we can put it into the nanoparticle. Then we will have to tune the nanoparticle to emit strong fluorescence, and also control the release of the drug into the tumour and not the bloodstream. But we are confident we can do all that,” Yang says.
Story Source:
The above story is based on materials provided by Penn State Materials Research Institute.