Credit: Image courtesy of blinktbi.
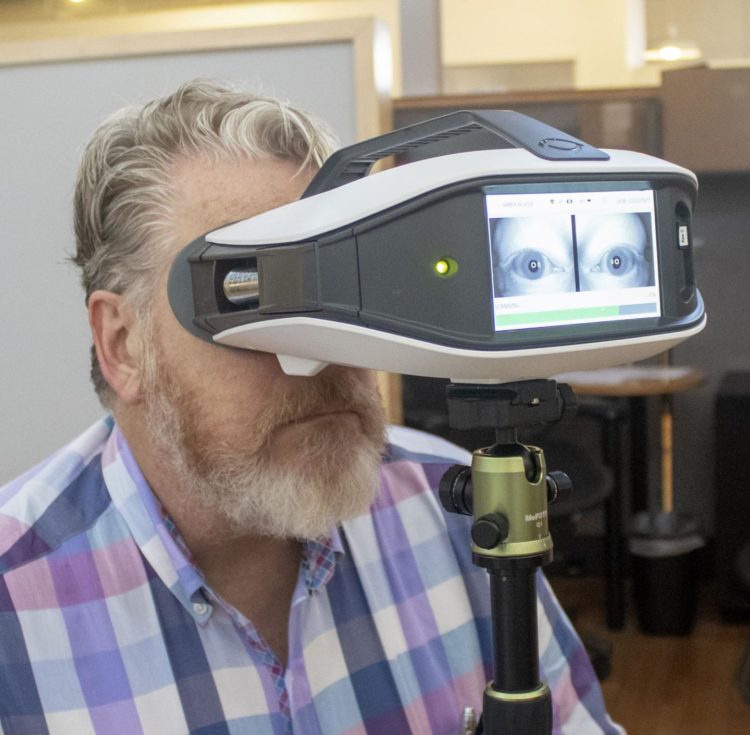
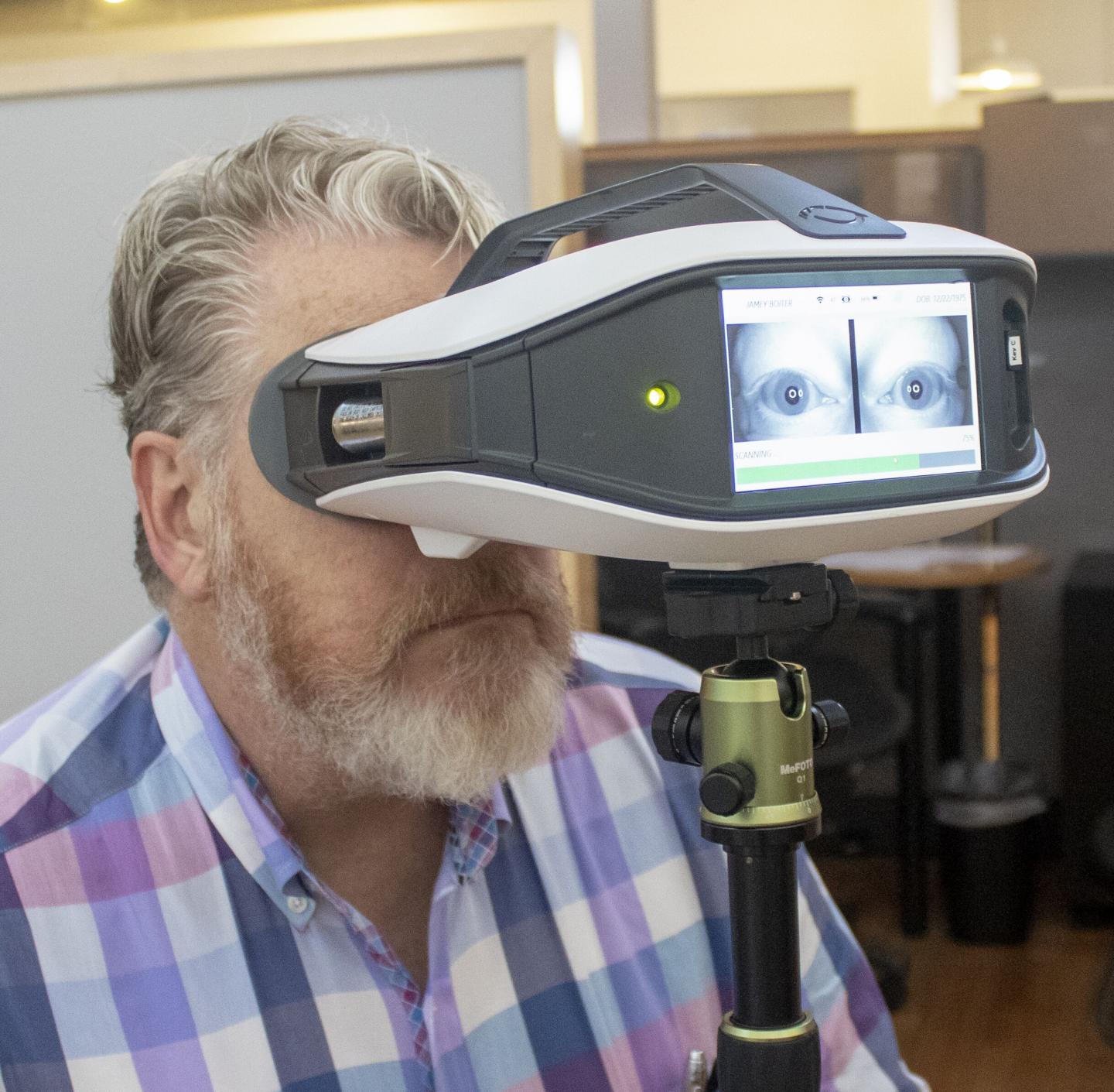
EyeStat (blinktbi, Charleston, SC), a portable, lightweight device for measuring the natural blink reflex, received clearance by the U.S. Food and Drug Administration (FDA) in December 2019.
The blink reflex activates when sensory or mechanical stimuli come into proximity to parts of the eye. These stimuli trigger certain nerves that send a signal through our brain and then back out telling us to blink. Ophthalmologists can use the blink reflex in the clinic to test for a variety of diseases that could cause these nerves or their communication to the brain to be damaged, and neurologists can use them to evaluate brain function.
To elicit the blink reflex, EyeStat delivers five light puffs of food-grade carbon dioxide at random intervals to the corner of the eyes over a 20-second time frame. During those 20 seconds, the device takes over 12,000 pictures to record the natural blink reflex. It specifically measures five things: the time it takes from the moment the air touches the side of the face until the eyelid on the stimulated side begins moving; the lag time between when the stimulated eye begins to move and the non-stimulated eye starts moving; how long the eye stays closed during a blink; the number of blinks immediately after the stimulus; and the distance between the place your eyelid sits when you are simply looking forward and when it is closed.
The FDA clearance enables blinktbi to market EyeStat as a means to measure and display the mechanically induced blink reflex, but not yet as a diagnostic device for particular clinical conditions.
However, blinktbi is currently researching whether the portable device could be used to clinically assess relevant changes in the blink reflex in patients subjectively diagnosed with traumatic brain injury on the playing field or the battlefield.
“Our clinical trials show our blink reflex changes with any trauma to the brain,” said Ryan Fiorini, Ph.D., blinktbi co-founder and chief operations officer.
The idea of creating a more maneuverable device for measuring the blink reflex originated from Nancey Tsai, M.D., an assistant professor in the Department of Neurosurgery at MUSC. Tsai wanted to create a portable blink “Reflexometer” to measure the blink reflex of athletes on the sidelines during games to monitor for concussions. She worked with the Zucker Institute for Applied Neurosciences (ZIAN), a technology incubator embedded in MUSC, to take the first steps towards producing a research device that could eventually be commercialized by a company such as blinktbi.
ZIAN filed a patent on the idea back in 2013 and began to build a prototype, collaborating with Lt. Colonel Dena Garner, Ph.D., professor in the Department of Health and Human Performance and director of undergraduate research at The Citadel, to collect initial data. Garner’s promising findings led ZIAN next to license the rights to blinktbi to develop, test and eventually commercialize the technology.
“We built some devices that could do preliminary testing; however, they were large and had to be wheeled around on carts and had no use as an on-field device. Thus, they weren’t very practical,” said Mark Semler, co-inventor of the device and ZIAN chief executive officer. “Blinktbi was able to shrink the device down to a small portable device designed for sideline or clinic use. They also significantly expanded the research and testing and obtained FDA clearance for the EyeStat device.”
EyeStat is being sold to high school, collegiate and professional sports teams around the country to measure the mechanically induced blink reflex. These devices “in the field” are helping blinktbi to collect the data needed to assess whether the device can effectively diagnose concussion.
However, the research being done by blinktbi does not stop there, according to Fiorini. “We are also excited to say that now that we have FDA clearance for the blink reflex, we are beginning multiple studies looking at indications in a number of different fields,” said Fiorini. “These include testing for sobriety and early onset of neurological diseases, such as multiple sclerosis and Alzheimer’s disease.”
All of these applications require more study and each would require separate FDA Clearance.
But the recent limited indication for EyeStat for measuring the mechanically induced blink response is an important milestone for the technology. It enables the device to be sold and used for the first time outside of a research study. The approval is the culmination of work by its inventors, the scientists who tested it and the entrepreneurs who have worked to commercialize it. With the FDA approval, the device has cleared an important hurdle, meaning that an idea that began at MUSC is closer to benefitting the nation.
###
About blinktbi
Blinktbi is a medical device company that has developed the world’s first FDA-cleared technology that measures and assesses the blink reflex. The game-changing technology, called EyeStat™, delivers objective data about the blink reflex in under one minute, helping medical professionals make better informed clinical decisions in real time. The patented device stimulates, measures and displays the blink reflex using light puffs of air and high speed videography in a 20 second test. EyeStat™ is non-invasive, lightweight and completely portable. For more information on EyeStat™, visit https:/
About the Medical University of South Carolina
Founded in 1824 in Charleston, the Medical University of South Carolina (MUSC) is the oldest medical school in the South, as well as the state’s only integrated, academic health sciences center with a unique charge to serve the state through education, research and patient care. Each year, MUSC educates and trains more than 3,000 students and 700 residents in six colleges: Dental Medicine, Graduate Studies, Health Professions, Medicine, Nursing and Pharmacy. The state’s leader in obtaining biomedical research funds, in fiscal year 2018, MUSC set a new high, bringing in more than $276.5 million. For information on academic programs, visit http://musc.
As the clinical health system of the Medical University of South Carolina, MUSC Health is dedicated to delivering the highest quality patient care available while training generations of competent, compassionate health care providers to serve the people of South Carolina and beyond. Comprising some 1,600 beds, more than 100 outreach sites, the MUSC College of Medicine, the physicians’ practice plan and nearly 275 telehealth locations, MUSC Health owns and operates eight hospitals situated in Charleston, Chester, Florence, Lancaster and Marion counties. In 2019, for the fifth consecutive year, U.S. News & World Report named MUSC Health the No. 1 hospital in South Carolina. To learn more about clinical patient services, visit http://muschealth.
MUSC and its affiliates have collective annual budgets of $3 billion. The more than 17,000 MUSC team members include world-class faculty, physicians, specialty providers and scientists who deliver groundbreaking education, research, technology and patient care.
About the Zucker Institute for Applied Neurosciences
The Zucker Institute for Applied Neurosciences (ZIAN) is a non-profit technology accelerator embedded within the Medical University of South Carolina (MUSC) Department of Neurosurgery in Charleston, S.C. Established in 2013, ZIAN’s vision is “to bring life-saving technology into the world by bridging the gap between clinical innovation and technical expertise.” ZIAN’s team includes a renowned faculty of clinicians and scientists, along with talented engineers and business development experts. The team collaborates with industry partners to swiftly and efficiently meet the increasing patient and practitioner demand for breakthroughs in the medical device space. ZIAN is currently developing technologies in the areas of spine, orthopedic and general surgery, imaging, dental, cardiovascular and neurovascular interventions. To learn more about ZIAN, call 843-792-5406 or visit ianeuro.org.
Media Contact
Heather Woolwine
[email protected]
843-792-7669