Credit: Osaka University
Osaka, Japan – A team at Osaka University has created single-molecule nanowires, complete with an insulation layer, up to 10 nanometers in length. When they measured the electrical properties of these nanowires, the researchers found that forcing the ribbon-like chains to be flat significantly improved their conductivity compared with a twisted conformation. The findings may allow for a new generation of inexpensive high-tech devices, including smartphone screens and photovoltaics.
Carbon-based polymers, which are long molecular chains made of repeating units, can be found everywhere, from the rubber in the soles of your shoes to the proteins that make up your body. We used to think that these molecules could not conduct electricity, but that all changed with the discovery of conducting polymers. These are a small subset of carbon-based molecules that can act like tiny wires owing to their alternating single and double chemical bonds, also called conjugated bonds. Since carbon-based conductors are much easier and cheaper to make and customize than conventional electronics, they have seen rapid adoption in OLED TVs, iPhone screens, and solar panels, while drastically reducing their cost.
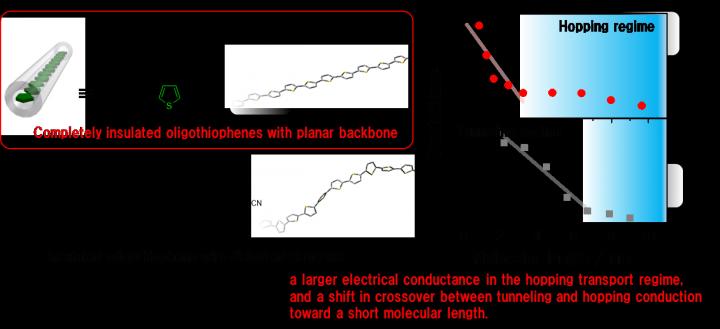
Now, researchers at Osaka University have synthesized chains of oligothiophene of various lengths, with up to 24 repeat units. This means that single nanowires could be up to 10 nanometers in length. Insulation of the wires was needed to avoid interwire currents, so that the intrinsic conductivity of a single molecule could be measured accurately. On the basis of the rules of quantum mechanics, electrons in molecules behave more like spread-out waves than localized particles. The overlapping bonds in oligothiophene allow electrons to be entirely spread out over the polymer backbone, so they can easily transverse the molecule to create an electrical current.
This charge transport can occur is two very different ways. “Over short distances, electrons rely on their wave-like nature to ‘tunnel’ directly through barriers, but over long distances, they hop from site to site to reach their destination,” first author Dr. Yutaka Ie explained. The team at Osaka University found that changing the oligothiophene chain from twisted to flat led to much greater overlap of the conjugated backbone of oligothiophene, which in turn meant a larger overall conductivity. As a result, the crossover from tunneling to hopping conduction took place with flat chains at shorter chain lengths, compared with those with the twisted conformation.
The researchers believe that this work can open a whole new world of devices. “This study demonstrates that our insulated nanowires have the potential to be used in novel ‘single-molecule’ electronics,” lead author Dr. Yoshio Aso said. The work is published in The Journal of Physical Chemistry Letters as “Highly Planar and Completely Insulated Oligothiophenes: Effects of π-Conjugation on Hopping Charge Transport.” (DOI: 10.1021/acs.jpclett.9b00747)
###
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




