Credit: Mitsuharu Okutsu
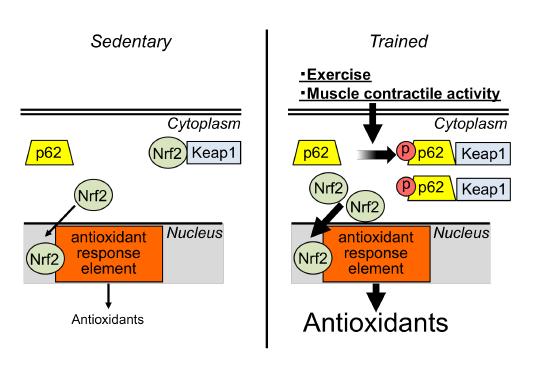
Skeletal muscle mass and function becomes progressively compromised with aging, chronic disease, and physical inactivity. Oxidative stress plays an important role in loss of skeletal muscle mass and function. Therefore, increased antioxidant expression could be a potentially protective measure against oxidative stress and skeletal muscle atrophy. Although muscle contractile activity, as observed with regular exercise, prevents oxidative stress-induced muscle wasting, at least partially by improving the antioxidant defense system, the regulation of antioxidant expression in skeletal muscle remains incompletely understood at the molecular level. In the present study, we hypothesized that regular endurance exercise enhances the expression of p62/SQSTM1 (p62) and its phosphorylation at Ser 351 thereby dissociating nuclear factor erythroid 2-related factor 2 (Nrf2) from Keap1, which results in increased Nrf2 nuclear translocation and DNA binding activity. Our major novel observations are: i) Enhanced muscle contractile activity increases antioxidants, nuclear translocation of Nrf2, and Nrf2 DNA-binding activity in mouse skeletal muscle. ii) Skeletal muscle expression of the Nrf2 target antioxidant gene NAD(P)H-quinone oxidoreductase 1 (NQO1) is abolished in both muscle-specific Nrf2 knockout (Nrf2 mKO) and muscle-speicifc p62 knockout (p62 mKO) mice. iii) Regular endurance exercise increases total p62 and Ser 351 phosphorylation of p62 in the oxidative soleus muscle. iv) Loss of Nrf2 (i.e, in Nrf2 mKO mice) or p62 (i.e., in p62 mKO mice) prevents exercise-mediated increase in the essential antioxidant proteins CuZnSOD and EcSOD in the oxidative soleus muscle. Collectively, these findings indicate that p62 and Nrf2 are cooperatively essential for regular exercise-mediated increase of antioxidant protein expression in oxidative muscle and suggest that Ser 351 phosphorylation of p62 plays a critical role in this regulation.
###
Media Contact
Mitsuharu Okutsu
[email protected]




