Cells that have undergone EMT can promote tumor growth and neovascularization either indirectly, by promoting endothelial transdifferentiation of carcinoma cells, or directly, by acquiring an endothelial phenotype, with FOXC2 playing key roles in these pr
Credit: Correspondence to – Tapasree Roy Sarkar – [email protected]
Oncotarget published “Carcinoma cells that have undergone an epithelial-mesenchymal transition differentiate into endothelial cells and contribute to tumor growth” which reported that the authors investigated whether EMT can confer endothelial attributes upon carcinoma cells, augmenting tumor growth and vascularization.
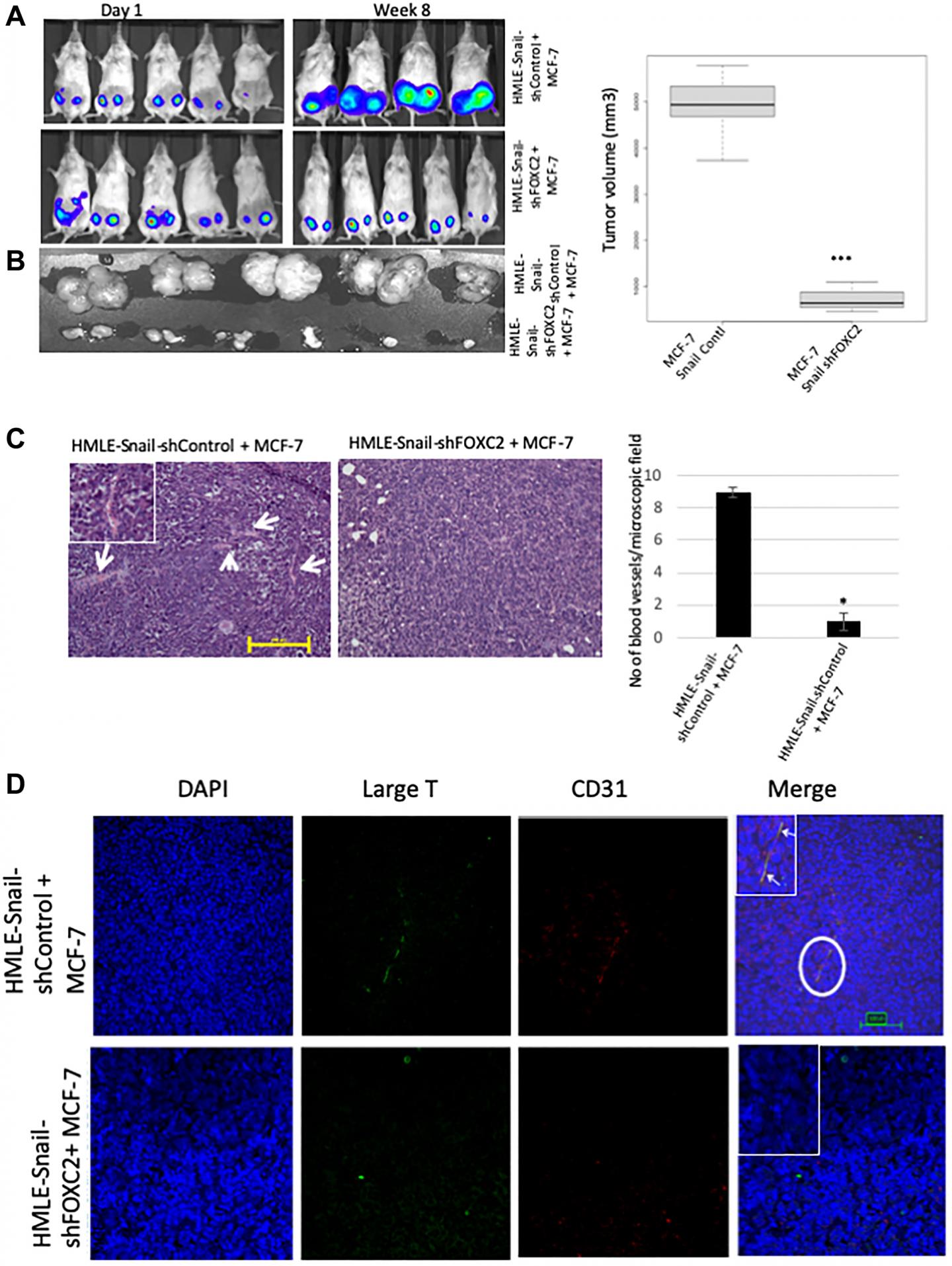
Hypoxic regions, demarcated by HIF-1α staining, exhibited focal areas of E-cadherin loss and elevated levels of vimentin and the EMT-mediator FOXC2. Implantation of MCF-7 cells, co-mixed with human mammary epithelial cells overexpressing the EMT-inducer Snail, markedly potentiated tumor growth and vascularization, compared with MCF-7 cells injected alone or co-mixed with HMLE-vector cells.
Intra-tumoral vessels contained CD31-positive cells derived from either donor cell type.
FOXC2 knockdown abrogated the potentiating effects of HMLE-Snail cells on MCF-7 tumor growth and vascularization, and compromised endothelial transdifferentiation of mesenchymal cells cultured in endothelial growth medium.
Hence, cells that have undergone EMT can promote tumor growth and neovascularization either indirectly, by promoting endothelial transdifferentiation of carcinoma cells, or directly, by acquiring an endothelial phenotype, with FOXC2 playing key roles in these processes.
Cells that have undergone EMT can promote tumor growth and neovascularization either indirectly, by promoting endothelial transdifferentiation of carcinoma cells, or directly, by acquiring an endothelial phenotype, with FOXC2 playing key roles in these processes
Dr. Tapasree Roy Sarkar from The University of Texas MD Anderson Cancer Center as well as The Texas A&M University said, “Angiogenesis is a normal physiological process that entails the development of new blood vessels through remodeling of a pre-existing vasculature, underpinned by endothelial cell sprouting, proliferation, and fusion“
A fourth mechanism—termed vasculogenic mimicry—entails the de novo generation of microvessels, lined with highly invasive tumor cells embedded in a rich extracellular matrix, essentially mimicking a true vascular endothelium and, notably, lacking in the endothelial cell markers CD31 and CD34.
Finally, newly formed blood vessels may emerge through transdifferentiation of neoplastic or tumor stem-like cells into CD31-positive endothelial-like cells, as has been documented in neuroblastoma, B-cell lymphoma, and glioblastoma.
In addition, subcutaneous injection of B16 melanoma cells into Foxc2 haploinsufficient mice has been shown to lead to the impaired formation of tumor blood vessels and, accordingly, compromise tumor growth.
Given the inherent plasticity of cells that have undergone EMT and the involvement of hypoxia in EMT and angiogenesis, the authors sought to ascertain whether cells, undergoing EMT in the hypoxic milieu, can acquire endothelial cell attributes and augment tumor growth by directly contributing to the tumor vasculature.
These findings findings link the stemness, conferred through EMT, to the acquisition of endothelial cell traits and the augmentation of tumor angiogenesis in a FOXC2-dependent manner.
The Sarkar Research Team concluded in their Oncotarget Research Output that their findings are consistent with the notion that the phenotypic attributes of cells within growing tumors are eminently pliable and that, as tumor size and the oxygen deficit increase, carcinoma cells become progressively dedifferentiated towards a mesenchymal, stem-like phenotype.
Indeed, they have previously used mathematical modeling to show that exposure to hypoxia augments the rate of dedifferentiation of non-stem, differentiated epithelial cells resulting in a shift towards a stem-like, plastic phenotype with increased EMT features.
On the basis of the findings herein, we propose that the induction of EMT contributes to tumor neoangiogenesis in two different ways:
Firstly, indirectly, by modifying the tumor niche, through paracrine signaling or alterations in extracellular matrix deposition, to promote the endothelial transdifferentiation of epithelial tumor cells.
Secondly, by generating stem-like cells that can assume endothelial-like phenotypic and functional attributes and directly integrate into the nascent tumor vasculature.
They also report that FOXC2, a common denominator of multiple EMT pathways, plays a vital role in the acquisition of endothelial phenotypic and functional characteristics in vitro and in vivo, with the corollary that inhibition of FOXC2 signaling may compromise tumor neoangiogenesis.
###
DOI – https:/
Full text – https:/
Correspondence to – Tapasree Roy Sarkar – [email protected]
Keywords –
angiogenesis,
endothelial transdifferentiation,
epithelial-mesenchymal transition,
vasculogenic mimicry,
FOXC2
About Oncotarget
Oncotarget is a bi-weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
To learn more about Oncotarget, please visit https:/
SoundCloud – https:/
Facebook – https:/
Twitter – https:/
LinkedIn – https:/
Pinterest – https:/
Reddit – https:/
Oncotarget is published by Impact Journals, LLC please visit https:/
Media Contact
RYAN JAMES JESSUP
[email protected]
Original Source
https:/
Related Journal Article
http://dx.



