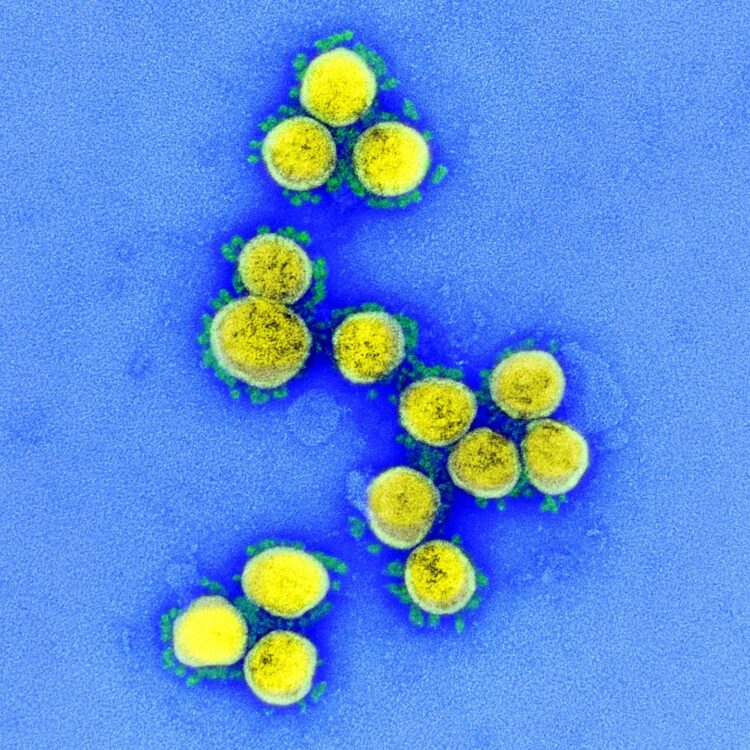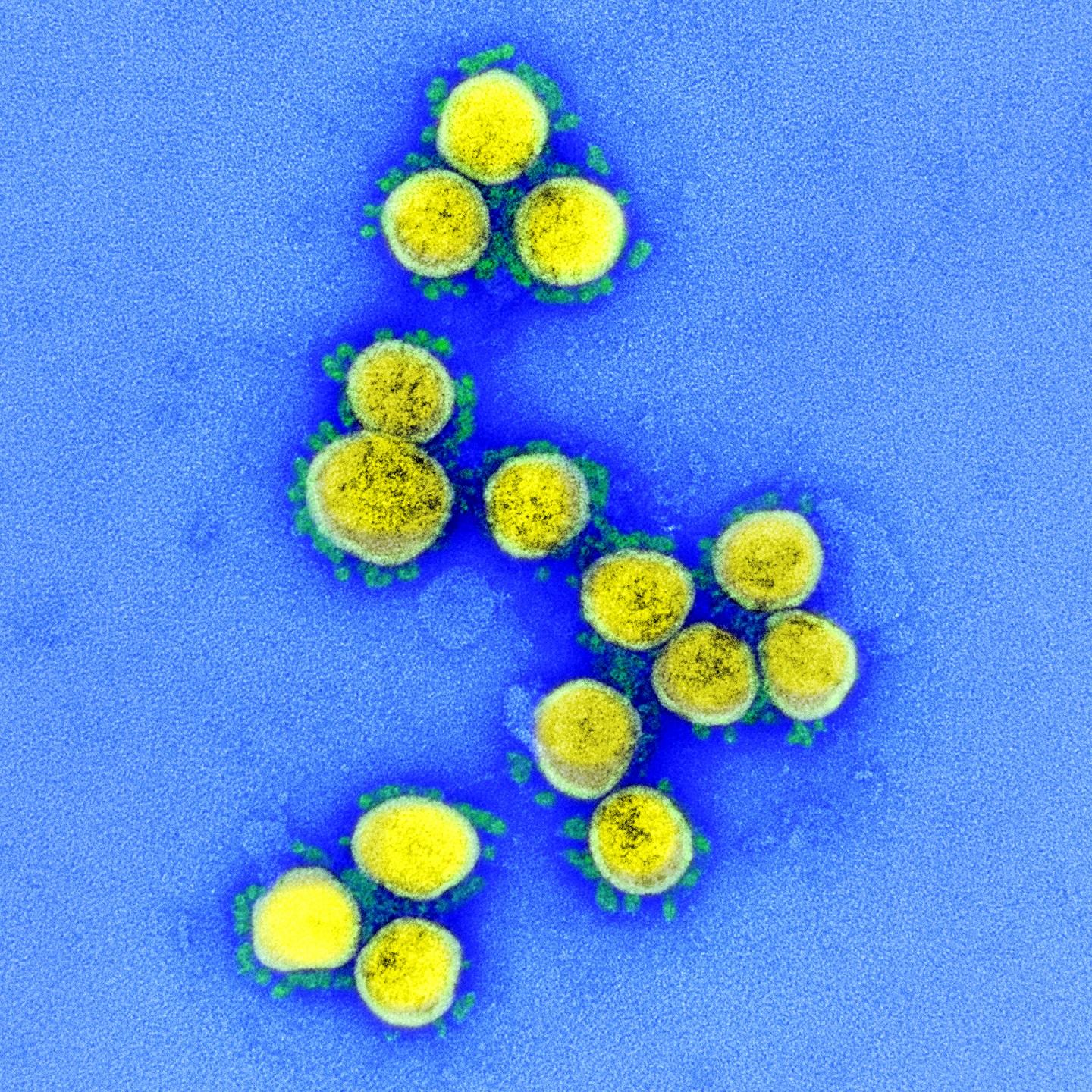
Credit: NIAID
A clinical trial to test the safety, tolerability and efficacy of a combination treatment regimen for coronavirus disease 2019 (COVID-19) consisting of the antiviral remdesivir plus a highly concentrated solution of antibodies that neutralize SARS-CoV-2, the virus that causes COVID-19, has begun. The study is taking place in hospitalized adults with COVID-19 in the United States, Mexico and 16 other countries on five continents. The National Institute of Allergy and Infectious Diseases (NIAID), part of the National Institutes of Health, is sponsoring and funding the Phase 3 trial, called Inpatient Treatment with Anti-Coronavirus Immunoglobulin, or ITAC.
The antibody solution being tested in the ITAC trial is anti-coronavirus hyperimmune intravenous immunoglobulin, or hIVIG. The antibodies in anti-coronavirus hIVIG come from the liquid portion of blood, or plasma, donated by healthy people who have recovered from COVID-19. These antibodies are highly purified and concentrated so that the anti-coronavirus hIVIG consistently contains several times more SARS-CoV-2 neutralizing antibodies than typically found in the plasma of people who have recovered from COVID-19.
The ITAC investigators hypothesize that giving people anti-coronavirus hIVIG at the onset of COVID-19 symptoms, before the body makes a protective immune response on its own, could augment the natural antibody response to SARS-CoV-2, thereby reducing the risk of more serious illness and death.
“Finding safe and effective treatments for COVID-19 is absolutely critical,” said NIAID Director Anthony S. Fauci, M.D. “The ITAC trial will examine whether adding anti-coronavirus hIVIG to a remdesivir regimen can give the immune system a needed boost to suppress SARS-CoV-2 early in the course of illness, nipping the infection in the bud.”
Leading the ITAC trial is Protocol Chair Mark Polizzotto, M.D., Ph.D., head of the Therapeutic and Vaccine Research Program at The Kirby Institute in the University of New South Wales, Sydney. The University of Minnesota is the coordinating center for the trial, which is being conducted by the NIAID-funded International Network for Strategic Initiatives in Global HIV Trials (INSIGHT). While INSIGHT was established to conduct clinical studies on HIV, it also has been involved in clinical trials related to influenza-like illness and the role of anti-influenza hIVIG since 2009. The ITAC trial also is known as INSIGHT 013.
Four companies are collaborating to provide anti-coronavirus hIVIG for the trial: Emergent BioSolutions of Gaithersburg, Maryland; Grifols S.A. of Barcelona; CSL Behring of King of Prussia, Pennsylvania; and Takeda Pharmaceuticals of Tokyo. The hIVIG from Emergent BioSolutions and Grifols S.A. was developed with support from the Biomedical Advanced Research and Development Authority, part of the Office of the Assistant Secretary for Preparedness and Response at the U.S. Department of Health and Human Services. CSL Behring and Takeda Pharmaceuticals are providing anti-coronavirus hIVIG on behalf of a partnership of plasma companies called the CoVIg-19 Plasma Alliance.
Remdesivir is currently recommended for treating certain hospitalized patients with COVID-19, based on an analysis of available data from the NIAID-sponsored Adaptive COVID-19 Treatment Trial (ACTT). ACTT found that hospitalized patients with COVID-19 and lower respiratory tract involvement who received remdesivir had a statistically significant shorter time to recovery compared to patients who received placebo. Remdesivir is an investigational broad-spectrum antiviral discovered and developed by Gilead Sciences, Inc. of Foster City, California.
The ITAC study team will enroll 500 hospitalized adults ages 18 or older who provide informed consent, have had COVID-19 symptoms for 12 days or fewer, and do not have life-threatening organ dysfunction or organ failure. Enrollment will occur at up to 58 sites in Africa, Asia, Europe, North America and South America. Study participants will be assigned at random to receive infusions of either anti-coronavirus hIVIG and remdesivir or a placebo and remdesivir. Neither the participants nor the study team will know who is receiving which treatment regimen.
hIVIG will be given as a single infusion of 400 milligrams (mg) per kilogram of current body weight. Remdesivir infusions will be administered as a 200-mg loading dose followed by a 100-mg once-daily intravenous maintenance dose during hospitalization for up to 10 days in total.
The main goal of the ITAC trial is to compare the health status of participants in the combination treatment group with participants in the remdesivir-only group on day 7. Health status will be based on an ordinal outcome with seven mutually exclusive categories ranging from no limiting symptoms due to COVID-19, to death. These categories capture the full range of severity experienced by hospitalized patients with COVID-19, according to the study investigators.
ITAC study participants will be followed for 28 days. If the trial goes to completion, the primary analysis will be completed after all participants finish 28 days of follow-up.
An independent data and safety monitoring board (DSMB) will review interim safety and efficacy data to ensure patient well-being and safety as well as study integrity.
The ITAC trial is associated with the Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV) public-private partnership. NIH and the Foundation for the NIH created ACTIV to develop a coordinated research strategy for prioritizing and speeding development of the most promising treatments and vaccines for COVID-19. ACTIV-associated trials are sponsored by NIH and have one or more industry partners. Both Gilead Sciences and Takeda Pharmaceuticals are ACTIV members.
Further information about the ITAC trial is available at ClinicalTrials.gov under study identifier NCT04546581.
NIAID conducts and supports research–at NIH, throughout the United States, and worldwide–to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH):
NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
Media Contact
NIAID Office of Communications
[email protected]
Original Source
https:/