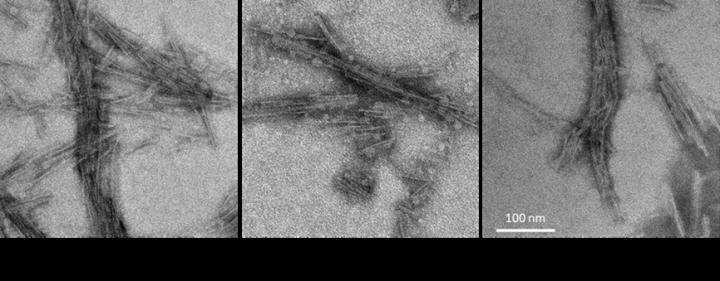
Samples of cerebrospinal fluid used to detect tauopathies
Credit: NIAID
WHAT:
National Institutes of Health (NIH) scientists have developed an ultrasensitive new test to detect abnormal forms of the protein tau associated with uncommon types of neurodegenerative diseases called tauopathies. As they describe in Acta Neuropathologica, this advance gives them hope of using cerebrospinal fluid, or CSF–an accessible patient sample–to diagnose these and perhaps other, more common neurological diseases, such as Alzheimer’s disease.
Scientists have linked the abnormal deposition of tau in the brain to at least 25 different neurodegenerative diseases. However, to accurately diagnose these diseases, brain tissue often must be analyzed after the patient has died.
For their study, the researchers used the same test concept they developed when using post-mortem brain tissue samples to detect the abnormal tau types associated with Pick disease, Alzheimer’s disease and chronic traumatic encephalopathy (CTE). They adapted the test to use CSF for the detection of abnormal tau of progressive supranuclear palsy (PSP), corticobasal degeneration (CBD), and other less common tauopathies.
They detected abnormal tau in CSF from both living and deceased patients. In one case, the test led to a corrected diagnosis in a patient who had died from CBD, but who was initially diagnosed with PSP. The new test is called 4R RT-QuIC–which stands for 4-repeat tau protein amplified in a real-time, quaking-induced conversion process.
The test was developed at NIH’s Rocky Mountain Laboratories, part of the National Institute of Allergy and Infectious Diseases. Study collaborators, who provided patient specimens and clinical guidance, included investigators from the Mayo Clinic in Jacksonville, Florida; the University of Bologna, Italy; the University of California, San Diego; the University of California, San Francisco; Indiana University; and the University of Verona in Italy.
The researchers plan to continue evaluating the clinical performance of 4R RT-QuIC by analyzing larger sets of CSF samples. One focus will be to compare test results from tauopathy patients who agree to provide CSF samples both before and after death. The scientists hope this type of evaluation will help them better understand how abnormal tau in CSF evolves during brain disease.
###
ARTICLE:
E. Saijo et al. 4-repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathologica DOI: 10.1007/s00401-019-02080-2 (2019).
WHO:
Byron Caughey, Ph.D., a senior investigator in NIAID’s Laboratory of Persistent Viral Diseases, is available to comment on this study.
CONTACT:
To schedule interviews, please contact Ken Pekoc, (301) 402-1663, [email protected].
NIAID conducts and supports research–at NIH, throughout the United States, and worldwide–to study the causes of infectious and immune-mediated diseases, and to develop better means of preventing, diagnosing and treating these illnesses. News releases, fact sheets and other NIAID-related materials are available on the NIAID website.
About the National Institutes of Health (NIH): NIH, the nation’s medical research agency, includes 27 Institutes and Centers and is a component of the U.S. Department of Health and Human Services. NIH is the primary federal agency conducting and supporting basic, clinical, and translational medical research, and is investigating the causes, treatments, and cures for both common and rare diseases. For more information about NIH and its programs, visit http://www.
NIH…Turning Discovery Into Health®
Media Contact
Ken Pekoc
[email protected]
301-402-1663
Related Journal Article
http://dx.




