Unusual structures in bacterial cells keep viral infection from spreading; a list of new ones could provide improved biotech tools
Credit: the Weizmann Institute of Science
Peculiar hybrid structures called retrons that are half RNA, half single-strand DNA are found in many species of bacteria. Since their discovery around 35 years ago, researchers have learned how to use retrons for producing single strands of DNA in the lab, but no one knew what their function was in the bacteria, despite much research into the matter. In a paper published today in Cell, a Weizmann Institute of Science team reports on solving the longstanding mystery: Retrons are immune system “guards” that ensure the survival of the bacterial colony when it is infected by viruses. In addition to uncovering a new strategy used by bacteria to protect themselves against viral infection — one that is surprisingly similar to that employed by plant immune systems — the research revealed many new retrons that may, in the future, add to the genome-editing toolkit.
The study, conducted in the lab of Prof. Rotem Sorek of the Institute’s Molecular Genetics Department, was led by Adi Millman, Dr. Aude Bernheim and Avigail Stokar-Avihail in his lab. Sorek and his team did not set out to solve the retron mystery; they were seeking new elements of the bacterial immune system, specifically elements that help bacteria to fend off viral infection. Their search was made easier by their recent finding that bacteria’s immune system genes tend to cluster together in the genome within so-called defense islands. When they uncovered the unique signature of retron within a bacterial defense island, the team decided to investigate further.
Their initial research showed that this retron was definitely involved in protecting bacteria against the viruses known as phages that specialize in infecting bacteria. As the researchers looked more closely at additional retrons located near known defense genes, they found that the retrons were always connected – physically and functionally – to one other gene. When either the accompanying gene or the retron was mutated, the bacteria were less successful in fighting off phage infection.
The researchers then set out to look for more such complexes in defense islands. Eventually, they identified some 5,000 retrons, many of them new, in different defense islands of numerous bacterial species.
To check if these retrons function, generally, as immune mechanisms, the researchers transplanted many retrons, one by one, into laboratory bacterial cells that were lacking retrons. As they suspected, in a great number of these cells they found retrons protecting the bacteria from phage infection.
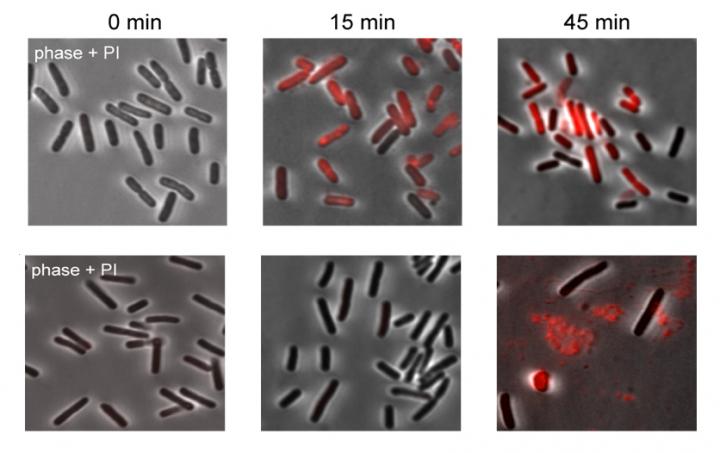
How do retrons do this? Focusing back on one particular kind of retron and tracing its actions in the face of phage infection, the research team discovered that its function is to cause the infected cell to commit suicide. Cell suicide, once thought to belong solely to multicellular organisms, is a last-ditch means of aborting widespread infection – if the suicide mechanism works fast enough to kill the cell before the virus finishes making copies of itself and spreading out to other cells.
Further investigation showed that retrons do not sense the phage invasion itself, but rather keep watch on another part of the immune system known as RecBCD, which is one of the bacterium’s first lines of defense. If it realizes that the phage has tampered with the cell’s RecBCD, the retron activates its program through the second, linked genes to kill the infected cell and protect the rest of the colony.
“It’s a clever strategy, and we found it works in a similar way to a guard mechanism employed in plant cells,” says Sorek. “Just like viruses that infect plants, phages come equipped with a variety of inhibitors to block assorted parts of the cell immune response. The retron, like a guard mechanism known to exist in plants, does not need to be able to identify all possible inhibitors, just to have a handle on the functioning of one particular immune complex. Infected plant cells apply this ‘abortive infection’ method, killing off a small region of a leaf or root, in an effort to save the plant itself. Since most bacteria live in colonies, this same strategy can promote the survival of the group, even at the expense of individual members.”
Retrons are so useful to biotechnology because they begin with a piece of RNA, which is the template for the synthesis of the DNA strand. This template in the retron sequence can be swapped out for any desired DNA sequence and used, sometimes in conjunction with another tool borrowed from the bacterial immune toolkit – CRISPR – to manipulate genes in various ways. Sorek and his team believe that within the diverse list of retrons they identified may be hiding more than a few that could provide better templates for specific gene editing needs.
###
Prof. Rotem Sorek is Head of the Knell Family Center for Microbiology; his research is supported by the Willner Family Leadership Institute for the Weizmann Institute of Science; the Sagol Weizmann-MIT Bridge Program; the Schwartz/Reisman Collaborative Science Program; the Ben B. and Joyce E. Eisenberg Foundation; the Yotam Project; and the European Research Council.
The Weizmann Institute of Science in Rehovot, Israel, is one of the world’s top-ranking multidisciplinary research institutions. Noted for its wide-ranging exploration of the natural and exact sciences, the Institute is home to scientists, students, technicians and supporting staff. Institute research efforts include the search for new ways of fighting disease and hunger, examining leading questions in mathematics and computer science, probing the physics of matter and the universe, creating novel materials and developing new strategies for protecting the environment.
Media Contact
Yael Edelman
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




