Credit: Norihito Fukui
Nagoya University researchers have synthesized a unique molecule with a surprising property: it can absorb near infrared light. The molecule is made only of hydrogen and carbon atoms and offers insights for making organic conductors and batteries. The details were published in the journal Nature Communications.
Organic chemist Hiroshi Shinokubo and physical organic chemist Norihito Fukui of Nagoya University work on designing new, interesting molecules using organic, or carbon-containing, compounds. In the lab, they synthesized an aromatic hydrocarbon called methoxy-substituted as-indacenoterrylene. This molecule has a unique structure, as its methoxy groups are located internally rather than at its periphery.
“Initially, we wanted to see if this hydrocarbon demonstrated novel phenomena due to its unique structure,” says Fukui.
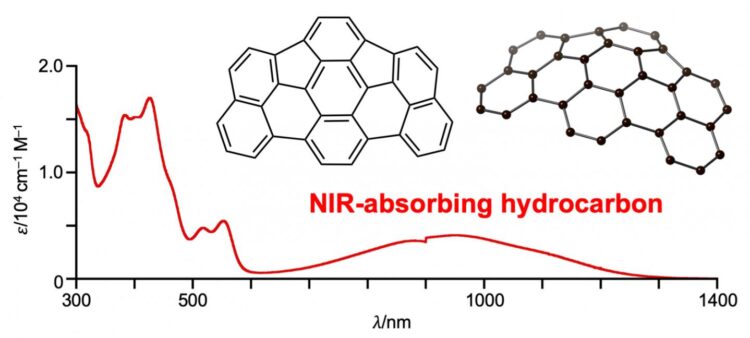
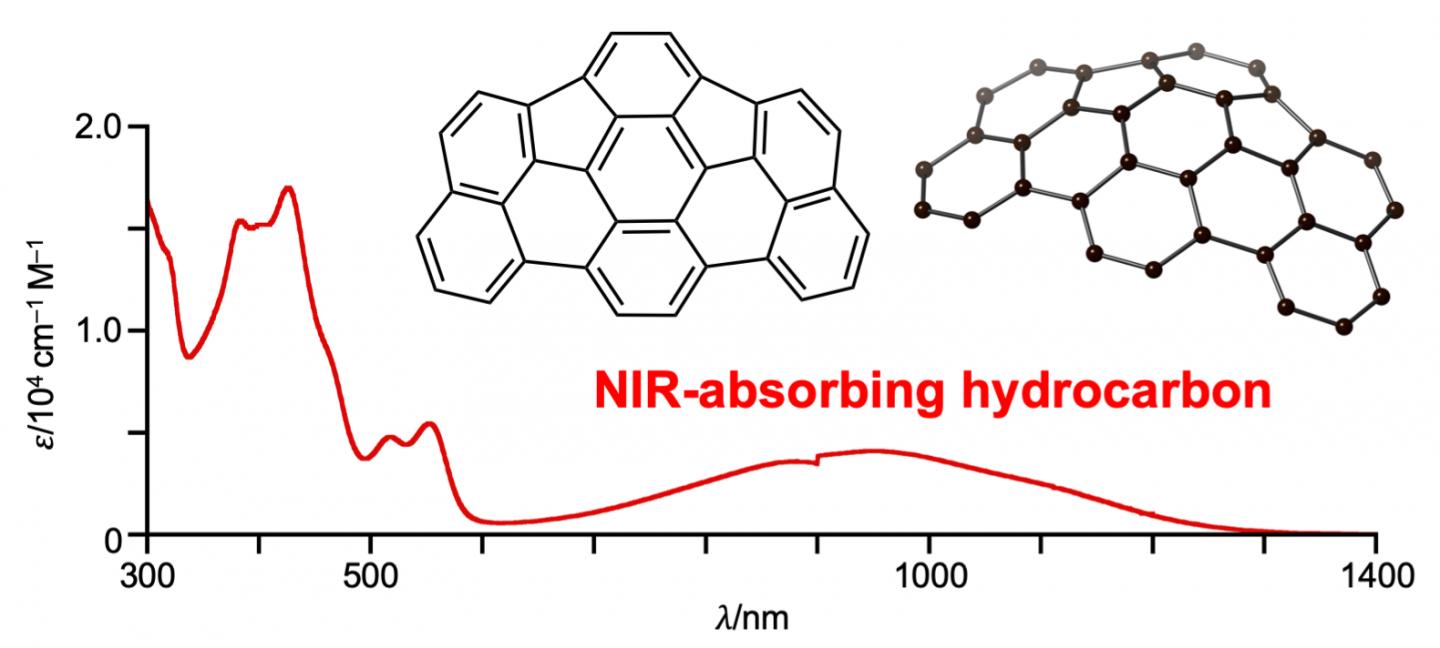
But during their investigations, the researchers discovered they could convert it into a new bowl-shaped hydrocarbon called as-indacenoterrylene.
“We were surprised to find that this new molecule exhibits near infrared absorption up to 1300 nanometers,” Shinokubo explains.
What’s unique about as-indacenoterrylene is not that it absorbs near infrared light. Other hydrocarbons can do this as well. as-indacenoterrylene is interesting because it does this despite being made of only 34 carbon and 14 hydrogen atoms, without containing other kinds of stabilizing atoms at its periphery.
When the scientists conducted electrochemical measurements, theoretical calculations, and other tests, they found that as-indacenoterrylene was intriguingly stable and also had a remarkably narrow gap between its highest occupied molecular orbital (HOMO) and its lowest unoccupied molecular orbital (LUMO). This means that the molecule has two electronically different subunits, one that donates and another that withdraws electrons. The narrow HOMO-LUMO gap makes it easier for electrons to become excited within the molecule.
“The study offers an effective guideline for the design of hydrocarbons with a narrow HOMO-LUMO gap, which is to fabricate molecules with coexisting electron-donating and electron-withdrawing subunits,” says Fukui. “These molecules will be useful for the development of next-generation solid-state materials, such as organic conductors and organic batteries.”
The team next plans to synthesize other near infrared-absorbing aromatic hydrocarbons based on the design concepts garnered in this current study.
###
This study, “as-Indaceno [3,2,1,8,7,6-ghijklm] terrylene as a near-infrared absorbing C70-fragment,” was published in the journal Nature Communications on August 3, 2020, at DOI: 10.1038/s41467-020-17684-6.
About Nagoya University, Japan
Nagoya University has a history of about 150 years, with its roots in a temporary medical school and hospital established in 1871, and was formally instituted as the last Imperial University of Japan in 1939. Although modest in size compared to the largest universities in Japan, Nagoya University has been pursuing excellence since its founding. Six of the 18 Japanese Nobel Prize-winners since 2000 did all or part of their Nobel Prize-winning work at Nagoya University: four in Physics – Toshihide Maskawa and Makoto Kobayashi in 2008, and Isamu Akasaki and Hiroshi Amano in 2014; and two in Chemistry – Ryoji Noyori in 2001 and Osamu Shimomura in 2008. In mathematics, Shigefumi Mori did his Fields Medal-winning work at the University. A number of other important discoveries have also been made at the University, including the Okazaki DNA Fragments by Reiji and Tsuneko Okazaki in the 1960s; and depletion forces by Sho Asakura and Fumio Oosawa in 1954.
Website: http://en.
Media Contact
Hiroshi Shinokubo
[email protected]
Original Source
http://en.
Related Journal Article
http://dx.