In the rapidly evolving field of cancer therapy, immunotherapy has emerged as a revolutionary approach, harnessing the innate power of the immune system to target and eliminate malignant cells. Despite its profound success stories in certain cancers like melanoma and lung carcinoma, this mode of treatment has yet to achieve equivalent breakthroughs in all tumor types. One such difficult-to-treat malignancy is tongue squamous cell carcinoma (TSCC), a prominent subtype of head and neck squamous cell carcinoma (HNSCC). Recent advances from the Institute of Science Tokyo shed light on the complex immune microenvironment of TSCC, offering hope for more personalized and effective interventions.
The challenge with TSCC immunotherapy arises from its unique tumor microenvironment, heavily influenced by the tongue’s constant exposure to myriad environmental and microbial stimuli. This ongoing interaction cultivates an immunotolerant niche, complicating efforts to rally an effective immune attack. Immune checkpoint inhibitors (ICIs), which function by blocking inhibitory pathways that stunt cytotoxic T lymphocyte (CTL) activity, have demonstrated only modest efficacy in TSCC. Clinical results reveal that durable responses appear in approximately 10% of treated patients, a stark contrast to the remarkable outcomes seen in other cancers.
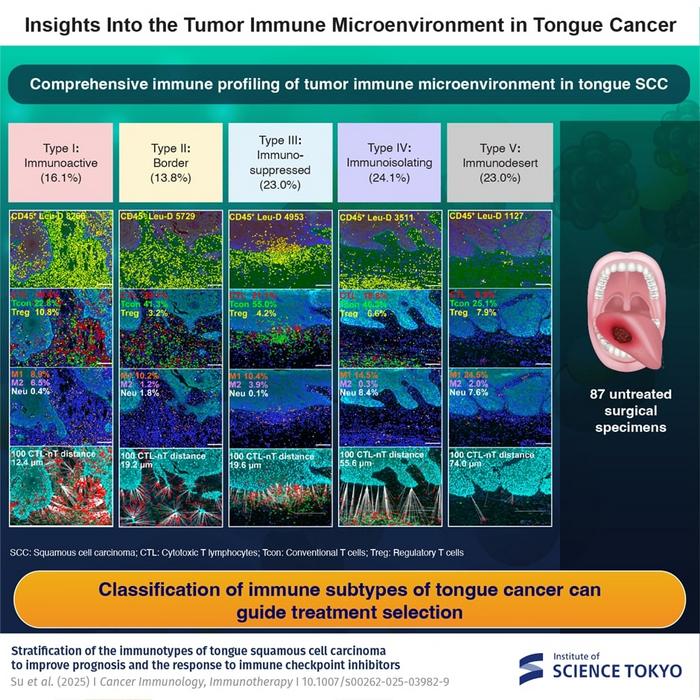
In an ambitious study led by Professor Miyuki Azuma, researchers have embarked on a comprehensive stratification of the immune landscape within TSCC tumors. Building on prior immune profiling datasets, their work, recently published in Cancer Immunology, Immunotherapy, uses cutting-edge multiplex immunofluorescence and sophisticated spatial tissue imaging techniques to reveal the nuanced interplay of immune subpopulations within the cancer microenvironment. Unlike traditional methods that broadly categorize tumors by size and metastatic stage, this immune-focused analysis uncovers pivotal distinctions in functional immune cell presence and distribution.
Key to their investigation was quantitative spatial analysis, measuring the physical proximity between tumor cells and cytotoxic T lymphocytes—immune cells integral to directly attacking and eradicating cancer. By mapping these interactions with exceptional precision, the study delineates five immunologically distinct TSCC subtypes, termed immunotypes. These vary from immunoactive environments harboring abundant and functionally engaged immune effector cells, to immunosuppressed and immunoisolating milieus where immune surveillance is thwarted or excluded. The fifth immunotype, immunodesert, is characterized by a near-total absence of immune cell infiltration, representing a particularly challenging therapeutic niche.
Among these categories, immunoactive type I tumors appear poised for a positive response to conventional ICI therapy due to their enriched immune cell activity and capacity for immune engagement. However, this promising profile is observed only in a minority of TSCC cases. The majority, approximately 70%, fall into immunosuppressed, immunoisolating, or immunodesert classifications—types that exhibit various mechanisms of immune evasion. Immunosuppressed type III, for instance, features active molecular pathways that directly inhibit immune cell function. Immunoisolating type IV tumors harbor immune cells that, despite infiltration, remain spatially or functionally disconnected from the malignant cells, impairing effective immune attack.
This stratification provides a compelling explanation for the historically low response rates to monotherapy with ICIs in TSCC. As Professor Azuma explains, “Our data demonstrate that most TSCCs do not present a conducive immune environment for checkpoint blockade alone to be successful. This underlines the necessity for therapeutic strategies tailored to the tumor’s immunological profile.” Crucially, this research highlights the limitations of traditional tumor-node-metastasis (TNM) staging, a system that fails to account for the tumor’s immune context and, consequently, lacks predictive power regarding immunotherapy outcomes.
The clinical implementation of immunotype classification stands to revolutionize personalized treatment planning. Patients exhibiting immunoactive tumors might benefit from existing ICI regimens as monotherapies. Conversely, those with immune-regulated types III through V would likely require combination approaches to overcome immune suppression or exclusion. Potential adjunct treatments could include molecular inhibitors targeting specific immunosuppressive pathways, adoptive cell therapies to enhance effector cell function, or agents aimed at reprogramming the tumor microenvironment to permit immune cell infiltration and engagement.
Technologically, the study’s deployment of multiplex immunofluorescence is a significant advance, allowing simultaneous visualization of multiple immune cell markers and functional proteins within a single tissue section. This multiplexing capability, paired with spatial analytics, provides a high-resolution map of the tumor’s immune milieu, facilitating precise characterization of immune cell subtypes, activation states, and their spatial relationships to tumor nests. Such data are indispensable for understanding the complex tumor-immune dynamics necessary to tailor effective immuno-oncological strategies.
This pioneering research also opens avenues for further basic and translational inquiry, including the exploration of molecular drivers underlying each immunotype, the genesis of immune exclusion or suppression, and the identification of biomarkers predictive of therapeutic response. By deepening insights into tumor-immune interactions in TSCC, the findings contribute to a broader understanding of immune resistance mechanisms that may be applicable across diverse cancer types as well.
Importantly, this study emerged from the newly established Institute of Science Tokyo, formed through the fusion of Tokyo Medical and Dental University and Tokyo Institute of Technology in late 2024. This interdisciplinary collaboration exemplifies the power of integrating cutting-edge biomedical science with technological innovation to tackle intractable clinical challenges.
The translation of immunotype-based classification into clinical practice promises to enhance precision oncology not only by predicting prognosis more accurately but also by guiding the rational design of combination therapies. As Professor Azuma concludes, “The future of TSCC treatment lies in stratifying patients by their tumor’s immune landscape, thus enabling selection of the most efficacious therapeutic regimens—a true embodiment of personalized medicine.”
With head and neck cancers remaining a global health challenge characterized by significant morbidity and mortality, the new immunological lens provided by this research marks a critical step towards refining treatment paradigms and ultimately improving patient survival and quality of life.
Subject of Research: Cells
Article Title: Stratification of the immunotypes of tongue squamous cell carcinoma to improve prognosis and the response to immune checkpoint inhibitors
News Publication Date: 1-Mar-2025
Web References: http://dx.doi.org/10.1007/s00262-025-03982-9
Image Credits: Institute of Science Tokyo, Japan
Keywords: Cancer, Cancer immunology, Oral cancer, Tongue, Immunology, Cells, Immune cells, Cell biology, Cancer genetics, Tumor regression, Tumor growth, Clinical medicine, Medical treatments, Cancer treatments, Cancer immunotherapy, Cancer research
Tags: advancements in oncology researchchallenges of immunotherapy in TSCCclinical outcomes of TSCC treatmentsenvironmental influences on tongue cancerimmune checkpoint inhibitors efficacyimmune landscape of tongue cancerimmune response in head and neck cancersimmunotherapy for tongue squamous cell carcinomamicrobial stimuli in TSCCpersonalized interventions for cancer therapytargeted therapies for squamous cell carcinomatumor microenvironment in HNSCC