Credit: FENG jiaqi
Electrochemical CO2 reduction reaction (CO2RR) is a promising approach to convert CO2 into useful chemicals.
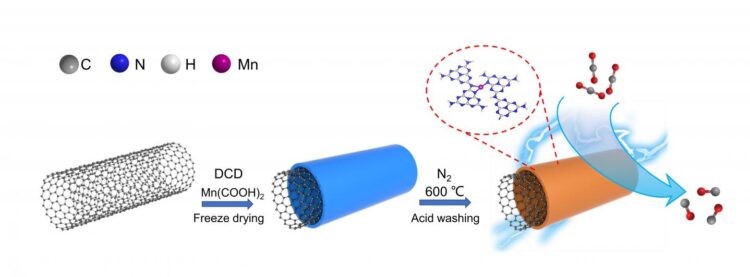
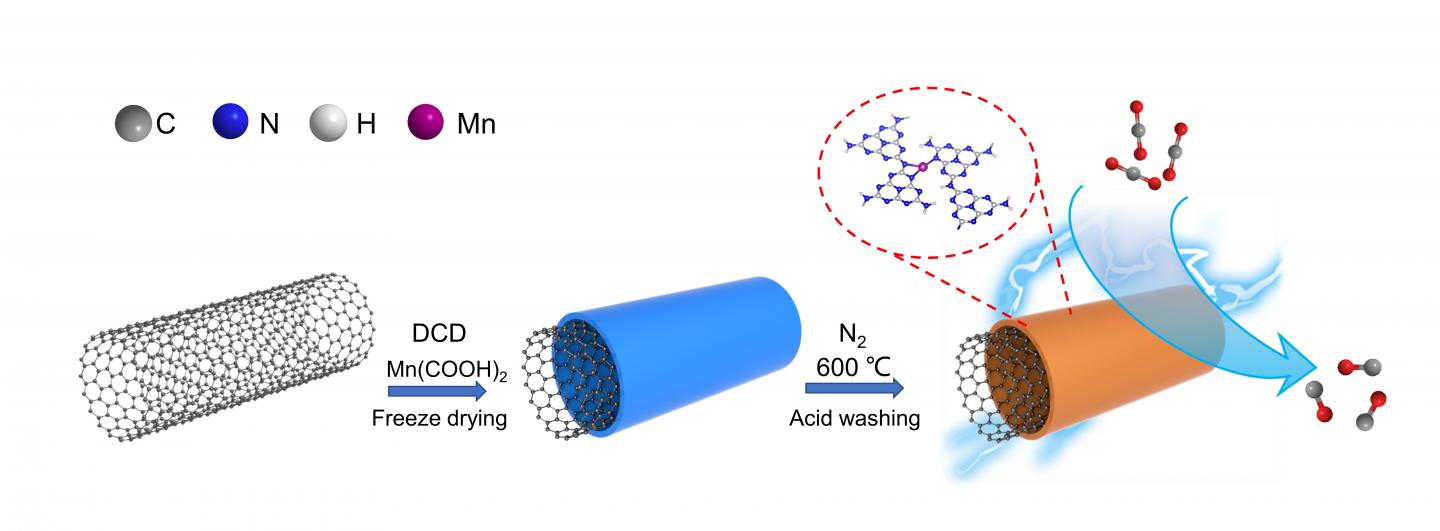
A research team led by Prof. ZHANG Suojiang from the Institute of Process Engineering (IPE) of the Chinese Academy of Sciences prepared a manganese (Mn) single-atom catalyst (SAC) with Mn-N3 site supported by graphitic C3N4, which exhibited efficient performance of CO2 electroreduction.
This work was published in Nature Communications on August 28.
It is a great challenge to obtain high Faradaic efficiency (FE) and high current density simultaneously by cheap catalysts for CO2RR.
The prepared catalyst exhibited a maximum CO FE of 98.8% with 14.0 mA cm-2 CO current density (jCO) at overpotential of 0.44 V in KHCO3 electrolyte, outperforming all reported Mn SACs.
Moreover, a higher jCO value of 29.7 mA cm-2 was obtained at overpotential of 0.62 V, when ionic liquid was used as electrolyte.
X-ray absorption spectroscopy and high-angle annular dark-field scanning transmission electron microscopy confirmed atomically dispersed Mn in the catalyst, and the best-fitting analysis indicated that the isolated Mn atom was three-fold coordinated by N atoms.
“In situ X-ray absorption spectra and density functional theory calculations demonstrated that the remarkable performance of the catalyst was attributed to the Mn-N3 site, which facilitated the formation of the key intermediate COOH* through a lowered free energy barrier,” said Prof. ZHANG Suojiang.
This work shows that the CO2RR activity of Mn-based catalysts can be enhanced through changing coordinated environment.
“It provides an important scientific basis and feasibility for low cost and high efficient electrochemical CO2 reduction to useful chemicals,” said Prof. ZHANG Xiangping, a co-corresponding author of the paper.
###
Media Contact
LI Xiangyu
[email protected]
Related Journal Article
http://dx.