Credit: Image created by K Lueckerath, UCLA.
Reston, VA–A combination of radionuclide therapy and immunotherapy has proven successful in slowing the progression of prostate cancer and increasing survival time, according to new research published in the February issue of The Journal of Nuclear Medicine. The results of the murine study indicate that radionuclide therapy promotes prostate cancer immunogenicity, provoking a cellular response that makes the tumors more receptive to immunotherapy.
“Prostate cancer is generally viewed as an immunological cold cancer in which immunotherapies only have moderate success,” said Katharina Lückerath, PhD, assistant professor of preclinical theranostics at the University of California Los Angeles in California. “Increasing prostate cancer immunogenicity with prostate-specific membrane antigen (PSMA) radionuclide therapy, however, might render immunotherapies more successful. In our research we sought to exploit this effect by combining radionuclide therapy with immunotherapy in a mouse model of prostate cancer.”
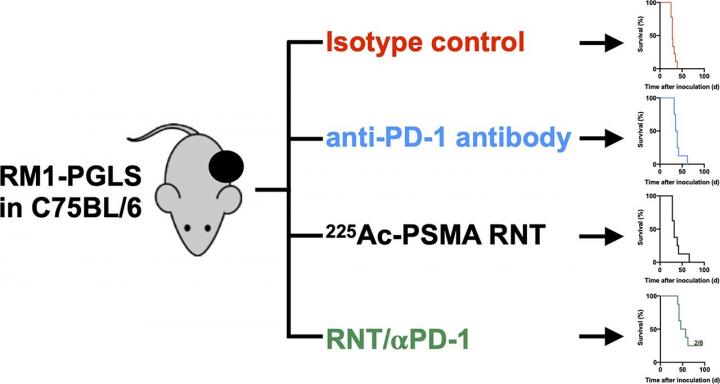
In the study, mice bearing prostate cancer tumors received one of four treatments: an isotope control antibody, 225Ac-PSMA617 radionuclide therapy, an anti-PD-1 antibody or both 225Ac-PSMA617 radionuclide therapy and an anti-PD-1 antibody. Therapeutic efficacy was assessed by measuring tumor volume (with computed tomography), time to progression and survival.
While 225Ac-PSMA617 radionuclide therapy and PD-1 blockade alone tended to prolong time to progression as compared to the isotope control (30 days and 33.5 days, respectively, versus 25 days), combining 225Ac-PSMA617 radionuclide therapy and PD-1 blockade significantly improved time to progression (47.5 days). Survival time also increased greatly for the joint therapeutic approach (51.5 days) when compared to225Ac-PSMA617 radionuclide therapy (32 days), anti-PD-1 (37 days) and the control (28 days).
“These findings suggest that radionuclide therapy may have a profound impact on the tumor immune microenvironment that can facilitate immunotherapies in prostate cancer,” noted Lückerath. “Radionuclide therapy and immunotherapy combinations are promising therapeutic options for prostate cancer patients, and the data reported in our study support the clinical translation of radionuclide therapy-immunotherapy combination regimens.”
In addition to this study, recently launched clinical trials for prostate cancer treatment and new tracers developed to image immune responses underscore the high interest in exploring immunity using molecular imaging and in combining radionuclide therapy with immunotherapy. According to Lückerath, these initiatives might expand the scope of nuclear medicine to immunology and strengthen nuclear medicine’s ability to offer powerful and versatile treatment options.
###
This study was made available online in July 2020 ahead of final publication in print in February 2021.
The authors of “Immune-Checkpoint Blockade Enhances 225Ac-PSMA617 Efficacy in a Mouse Model of Prostate Cancer” include Johannes Czernin, Kyle Current, Christine E. Mona, Lea Nyiranshuti, Firas Hikmat, Caius G. Radu and Katharina Lückerath, Department of Molecular and Medical Pharmacology, David Geffen School of Medicine, UCLA, Los Angeles, California.
This study was partially funded by the Prostate Cancer Foundation (19CHAL09). Johannes Czernin and Caius Radu are cofounders and hold equity in Sofie Biosciences, Trethera Therapeutics. Intellectual property has been patented by UCLA and licensed to Sofie Biosciences, Trethera Therapeutics, but was not used in the current study.
Please visit the SNMMI Media Center for more information about molecular imaging and precision imaging. To schedule an interview with the researchers, please contact Rebecca Maxey at (703) 652-6772 or [email protected].
About the Society of Nuclear Medicine and Molecular Imaging
The Journal of Nuclear Medicine (JNM) is the world’s leading nuclear medicine, molecular imaging and theranostics journal, accessed close to 10 million times each year by practitioners around the globe, providing them with the information they need to advance this rapidly expanding field. Current and past issues of The Journal of Nuclear Medicine can be found online at http://jnm.
JNM is published by the Society of Nuclear Medicine and Molecular Imaging (SNMMI), an international scientific and medical organization dedicated to advancing nuclear medicine and molecular imaging–precision medicine that allows diagnosis and treatment to be tailored to individual patients in order to achieve the best possible outcomes. For more information, visit http://www.
Media Contact
Rebecca Maxey
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.




