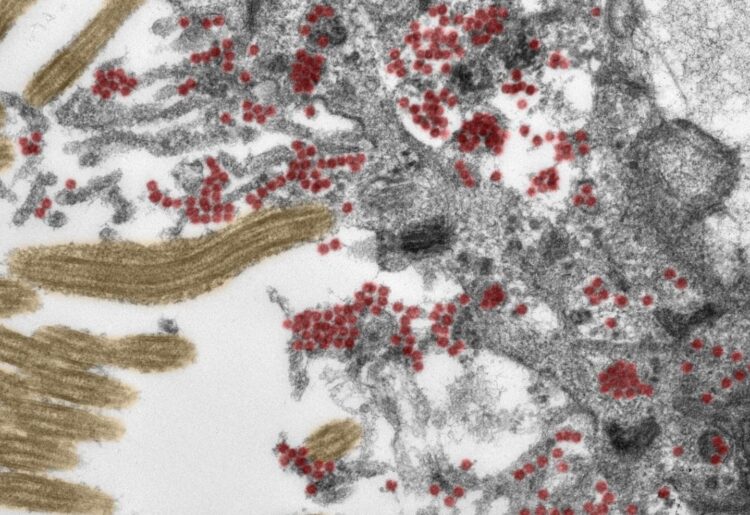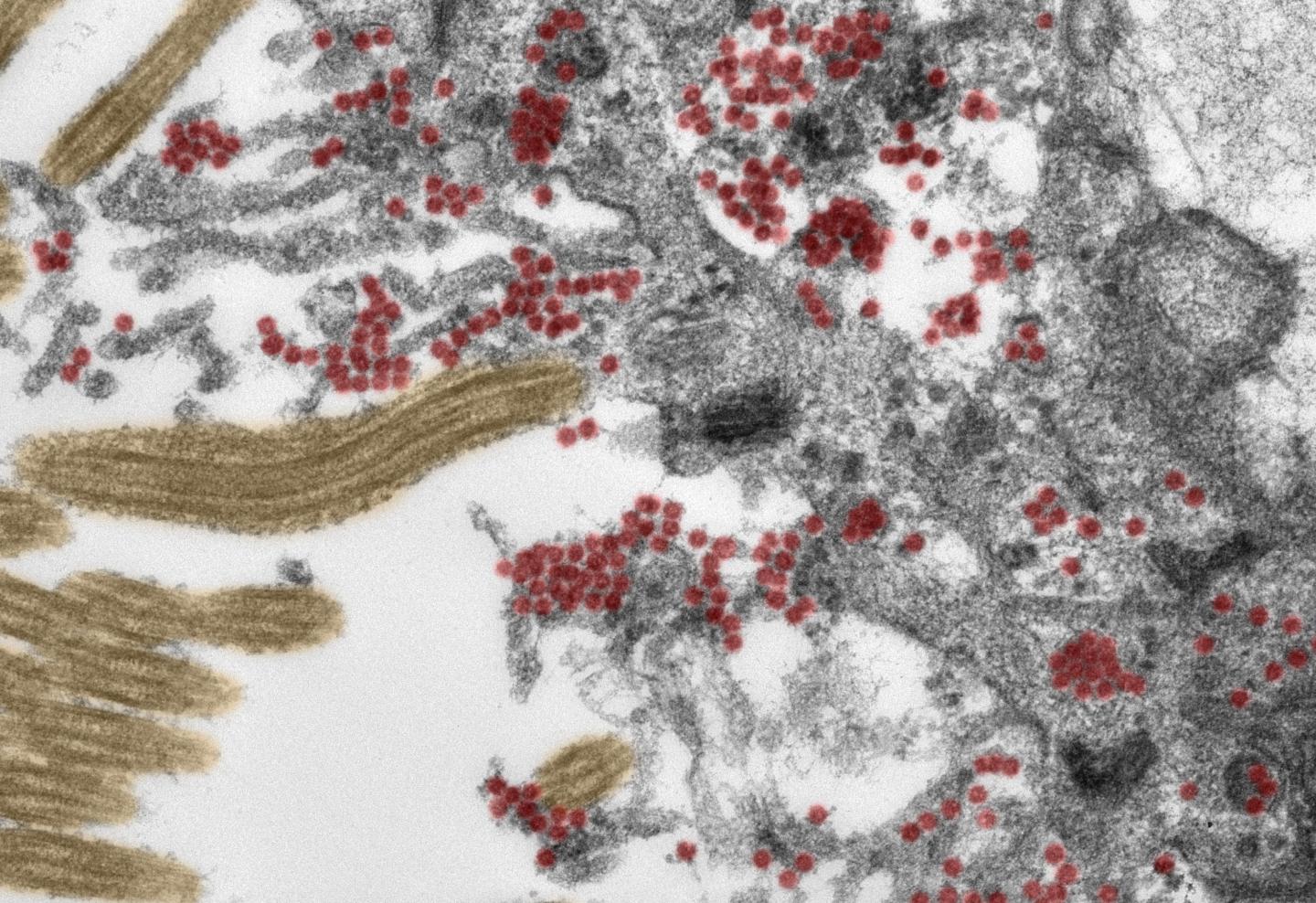
Credit: Photo: Michael Laue/RKI & Carsten Dittmayer/Charité
Using post-mortem tissue samples, a team of researchers from Charité – Universitätsmedizin Berlin have studied the mechanisms by which the novel coronavirus can reach the brains of patients with COVID-19, and how the immune system responds to the virus once it does. The results, which show that SARS-CoV-2 enters the brain via nerve cells in the olfactory mucosa, have been published in Nature Neuroscience*. For the first time, researchers have been able to produce electron microscope images of intact coronavirus particles inside the olfactory mucosa.
It is now recognized that COVID-19 is not a purely respiratory disease. In addition to affecting the lungs, SARS-CoV-2 can impact the cardiovascular system, the gastrointestinal tract and the central nervous system. More than one in three people with COVID-19 report neurological symptoms such as loss of, or change in, their sense of smell or taste, headaches, fatigue, dizziness, and nausea. In some patients, the disease can even result in stroke or other serious conditions. Until now, researchers had suspected that these manifestations must be caused by the virus entering and infecting specific cells in the brain. But how does SARS-CoV-2 get there? Under the joint leadership of Dr. Helena Radbruch of Charité’s Department of Neuropathology and the Department’s Director, Prof. Dr. Frank Heppner, a multidisciplinary team of researchers has now traced how the virus enters the central nervous system and subsequently invades the brain.
As part of this research, experts from the fields of neuropathology, pathology, forensic medicine, virology and clinical care studied tissue samples from 33 patients (average age 72) who had died at either Charité or the University Medical Center Göttingen after contracting COVID-19. Using the latest technology, the researchers analyzed samples taken from the deceased patients’ olfactory mucosa and from four different brain regions. Both the tissue samples and distinct cells were tested for SARS-CoV-2 genetic material and a ‘spike protein’ which is found on the surface of the virus. The team provided evidence of the virus in different neuroanatomical structures which connect the eyes, mouth and nose with the brain stem. The olfactory mucosa revealed the highest viral load. Using special tissue stains, the researchers were able to produce the first-ever electron microscopy images of intact coronavirus particles within the olfactory mucosa. These were found both inside nerve cells and in the processes extending from nearby supporting (epithelial) cells. All samples used in this type of image-based analysis must be of the highest possible quality. To guarantee this was the case, the researchers ensured that all clinical and pathological processes were closely aligned and supported by a sophisticated infrastructure.
“These data support the notion that SARS-CoV-2 is able to use the olfactory mucosa as a port of entry into the brain,” says Prof. Heppner. This is also supported by the close anatomical proximity of mucosal cells, blood vessels and nerve cells in the area. “Once inside the olfactory mucosa, the virus appears to use neuroanatomical connections, such as the olfactory nerve, in order to reach the brain,” adds the neuropathologist. “It is important to emphasize, however, that the COVID-19 patients involved in this study had what would be defined as severe disease, belonging to that small group of patients in whom the disease proves fatal. It is not necessarily possible, therefore, to transfer the results of our study to cases with mild or moderate disease.”
The manner in which the virus moves on from the nerve cells remains to be fully elucidated. “Our data suggest that the virus moves from nerve cell to nerve cell in order to reach the brain,” explains Dr. Radbruch. She adds: “It is likely, however, that the virus is also transported via the blood vessels, as evidence of the virus was also found in the walls of blood vessels in the brain.” SARS-CoV-2 is far from the only virus capable of reaching the brain via certain routes. “Other examples include the herpes simplex virus and the rabies virus,” explains Dr. Radbruch.
The researchers also studied the manner in which the immune system responds to infection with SARS-CoV-2. In addition to finding evidence of activated immune cells in the brain and in the olfactory mucosa, they detected the immune signatures of these cells in the cerebral fluid. In some of the cases studied, the researchers also found tissue damage caused by stroke as a result of thromboembolism (i.e. the obstruction of a blood vessel by a blood clot). “In our eyes, the presence of SARS-CoV-2 in nerve cells of the olfactory mucosa provides good explanation for the neurologic symptoms found in COVID-19 patients, such as a loss of the sense of smell or taste,” explains Prof. Heppner. “We also found SARS-CoV-2 in areas of the brain which control vital functions, such as breathing. It cannot be ruled out that, in patients with severe COVID-19, presence of the virus in these areas of the brain will have an exacerbating impact on respiratory function, adding to breathing problems due to SARS-CoV-2 infection of the lungs. Similar problems might arise in relation to cardiovascular function.”
###
*Meinhardt J et al., Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci 2020. doi: 10.1038/s41593-020-00758-5
On this study
This study would not have been possible without the consent of the patients and/or their family members. The authors are immensely grateful to them. Post-mortem examinations performed by neuropathologists and pathologists on patients who have died of COVID-19 require the same level of personal protective equipment that is used when dealing with individuals with e.g. HIV or tuberculosis. Results from this study were published as a preprint (prior to peer review) on 4 June 2020. Following completion of the peer review process, the paper has now been published in Nature Neuroscience.
Media Contact
Prof. Dr. Frank Heppner
[email protected]
Original Source
https:/
Related Journal Article
http://dx.