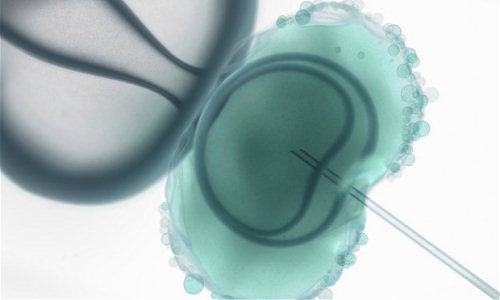
On February 25 and 26 the US Food and Drug Administration discussed the possibility of legalising three-parent embryos – or, in scientific lingo, “oocyte modification in assisted reproduction for the prevention of transmission of mitochondrial disease or treatment of infertility”.
This procedure, which involves removing the nucleus from one human egg whose cytoplasm contains defective mitochondria and placing it in an enucleated egg with healthy DNA for subsequent fertilisation, is also being debated in the UK.
The measure is strongly opposed by the Center for Genetics and Society, which is promoting an open letter to the FDA. It claims that mitochondrial transfer is unsafe, is effectively experimentation on unconsenting human subjects, and would only help a handful of women. Most importantly, it constitutes germline modification, a form of eugenics. This is a bright line which no country has ever stepped across.
Story Source:
The above story is based on materials provided by bioedge, Michael Cook.