Innovative method offers a new way of studying developmental cardiac biomechanics, live in 4D
Credit: Wang and Larina.
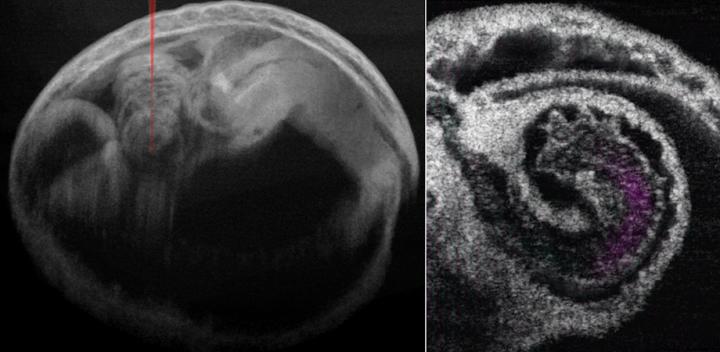
How a valveless embryonic heart tube pumps blood is a long-standing scientific mystery. Thanks to innovations in light-based technology, fresh insights are now available into the biomechanics of mammalian cardiogenesis—and in particular, the pumping dynamics of the mammalian tubular embryonic heart.
4D OCT (3D + time)
Shang Wang of the Stevens Institute of Technology and Irina Larina of the Baylor College of Medicine used cutting-edge 4D optical coherence tomography (OCT) to study the pumping mechanism underlying the developing mammalian heart for the first time. Their report, published in the peer-reviewed open access Journal of Biomedical Optics, demonstrates that 4D OCT imaging of mouse embryonic heart can provide unprecedented information about how the early mammalian heart works.
The study demonstrates the richness of data provided by this approach and its feasibility for investigating the functional relation between blood flow and heart wall dynamics within different regions of the embryonic mammalian heart—a possibility not currently accessible by other methods. The approach can be potentially used to assess cardiac pumping over embryonic development as the heart tube remodels, which could reveal functional changes during early cardiogenesis.
Biomechanics of the tiny mouse heart
The unique imaging scales and dynamic contrasts offered by OCT enable millimeter-level imaging depth with a microscale resolution that is ideal for capturing the entire mouse heart at mid-gestation stages. OCT also provides a clear view of fine cardiac structures as well as blood flow. The high imaging speed of OCT together with post-acquisition synchronization allows reconstructing the fast dynamics of the beating heart.
Amy L. Oldenburg, director of the Optical Coherence Imaging Laboratory at University of North Carolina at Chapel Hill, remarked, “The innovative method offers a new way of studying developmental cardiac biomechanics. Analysis of the 4D OCT images allowed Wang and Larina to relate blood flow, flow resistance, and pressure gradients induced by heart wall movements.”
There is much to be learned. Although the mechanism that pumps blood within the embryonic heart tube has traditionally been thought to be wavelike peristaltic contractions, Wang and Larina were able to offer a more detailed assessment using 4D OCT to integrate cardiodynamics and hemodynamics. Their pilot observations suggest that localized heart tube pumping in the ventricles functions through a combination of suction and pushing mechanisms.
Increasing understanding of congenital heart defects
Biomechanical factors are increasingly recognized for their essential roles in stimulating and regulating the heart development. The authors hope that their approach may inspire new ideas and innovative designs in imaging and measurement techniques to assess the embryonic cardiac biomechanics. In particular, the method may provide useful ways to better understand the mechanisms contributing to congenital heart defects, which are abnormal formations of the heart that develop before birth. According to Oldenburg, the results of this study “showcase the utility of these methods for studying biomechanical changes in mutant embryonic hearts that model congenital heart defects.” As mutant mouse lines modeling congenital heart defects are widely available, the method may contribute to increased understanding of the earliest development of the most common form of birth defect in humans.
Read the original open access report: “Live mechanistic assessment of localized cardiac pumping in mammalian tubular embryonic heart,” J. of Biomedical Optics, 25(8), 086001 (2020), doi 10.1117/1.JBO.25.8.086001
Media Contact
Daneet Steffens
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




