Credit: UNIST
A research team, led by Professor Guntae Kim in the School of Energy and Chemical Engineering at UNIST has introduced an innovative way to quantify proton kinetic properties of triple (H+/O2?/e?) conducting oxides (TCOs) being a significant indicator for characterizing the electrochemical behavior of proton and the mechanism of electrode reactions.
Layered perovskites have recently received much attention as they have been regarded as promising cathode materials for protonic ceramic fuel cells (PCFCs) that use proton conducting oxide (PCO) as an electrolyte. Therefore, quantitative characterization of the proton kinetics in TCO can be an important indicator providing a scientific basis for the rational design of highly efficient electrode materials of PCFCs, noted the research team.
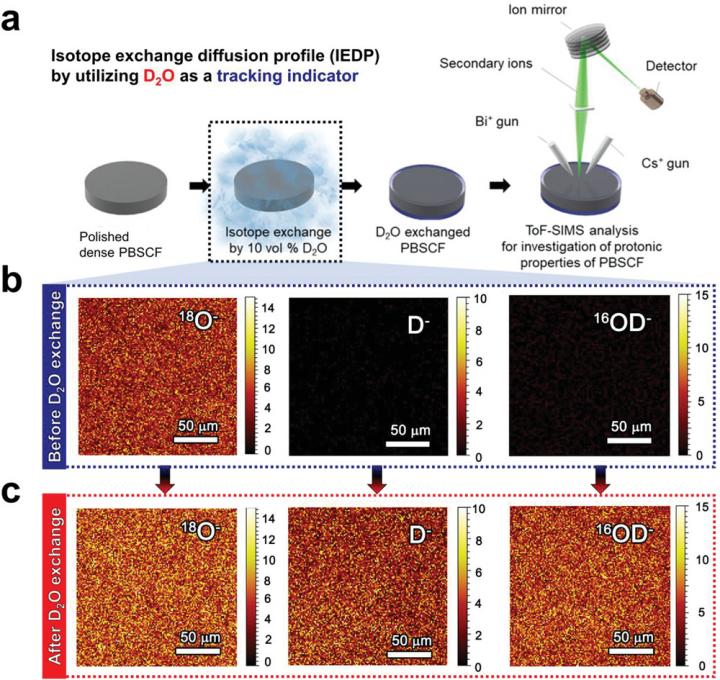
In this study, the research team employed the isotope exchange diffusion profile (IEDP) method to evaluate the proton kinetic properties of proton in the layered perovskite-type TCOs, PrBa0.5Sr0.5Co1.5Fe0.5O5+δ (PBSCF).
Using heavy water (deuterium oxide, D2O), as a tracking indicator of proton diffusion via time-of-flight secondary ion mass spectrometry (ToF-SIMS), the research team observed the proton formation and transport on both surface and bulk of the PBSCF. The findings revealed that the PBSCF showed two orders of magnitude higher proton tracer diffusion coefficient (D*H, 1.04 × 10?6 cm2 s?1 at 550 °C) than its oxygen diffusion coefficient at even higher temperature range (D*O, 1.9 × 10?8 cm2 s?1 at 590 °C). With the application of this fast proton transfer ability, the PBSCF cathode also exhibited excellent electrochemical performance for PCFC operation at low temperatures (e.g., 0.42 W cm?2 at 500 °C), which is bar far the best performance ever reported, noted the research team.
Meanwhile, a fuel cell is an eco-friendly energy conversion system that uses the chemical energy of hydrogen or another fuel to generate electricity. Among them, PCFCs have shown great potential to be operated at relatively low temperatures. Advantages of this class of fuel cells include a wide range of operating temperature and material choice, which could solve critical issues for solid-state electrochemical devices.
This research has been jointly carried out by Professor Sivaprakash Sengodan from UK’s Imperial College London, Professor Meilin Liu from the Georgia Institute of Technology in the United States, and Professor Sihyuk Choi from Kumoh National Institute of Technology. This study was made available online in March 2021 ahead of final publication in Advanced Science in June 2021.
###
Media Contact
JooHyeon Heo
[email protected]
Original Source
https:/




