Esophageal squamous cell carcinoma (ESCC) emerges as a significant health concern worldwide, characterized by its aggressive nature and poor prognosis despite available treatment options. The pressing need for innovative therapeutic strategies drives ongoing research into potential treatments for this prevalent cancer type. In recent studies, researchers have turned their attention to repurposing existing medications, with dronedarone—a drug initially approved for atrial fibrillation—showing promise in combating the proliferation of ESCC through inhibition of critical cellular pathways.
Dronedarone’s potential was scrutinized through a comprehensive screening process involving various FDA-approved drugs aimed at identifying candidates that could halt ESCC progression. Among the tested compounds, dronedarone stood out as a powerful inhibitor of ESCC cell growth. Subsequent laboratory experiments revealed that dronedarone markedly diminished cell proliferation in ESCC cultures while sparing non-cancerous esophageal epithelial cells from cytotoxic effects. This selective toxicity enhances the appeal of dronedarone as a therapeutic agent targeting tumor cells.
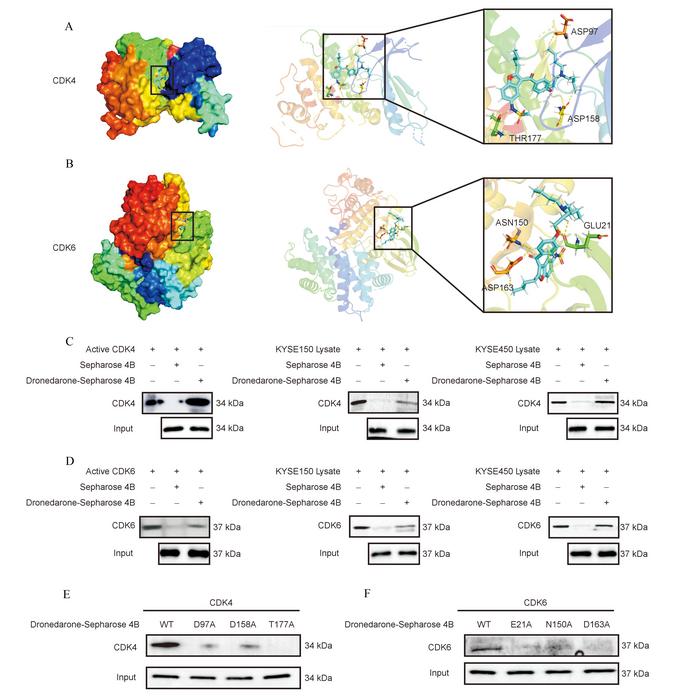
To understand the underlying mechanisms by which dronedarone exerts its effects, researchers employed proteomic analyses to investigate alterations in cellular signaling post-treatment. These investigations revealed significant downregulation of phosphorylation sites on the retinoblastoma protein 1 (RB1), indicating that dronedarone interferes with the CDK4/CDK6-RB1 axis, a critical regulator of the cell cycle. This interaction provides a framework for understanding how dronedarone acts to impede cancer cell progression, leading to cell cycle arrest at the G1 phase, a critical checkpoint in cellular replication.
Further validating the role of CDK4 and CDK6 in ESCC, scientists employed CRISPR technology to knockout these kinases in ESCC cell lines. The results were telling; the absence of CDK4/CDK6 drastically reduced cellular proliferation, establishing these enzymes as key players in ESCC biology and potential therapeutic targets. Interestingly, knockout ESCC cells exhibited increased resistance to dronedarone treatment, spotlighting the necessity of the CDK4/CDK6 pathway in mediating the drug’s efficacy.
The promise of dronedarone extends beyond in vitro findings. Utilizing patient-derived xenograft (PDX) models in immunocompromised mice allowed researchers to evaluate the therapeutic potential of dronedarone in vivo. The results were compelling—dronedarone treatment led to significant reductions in tumor size and weight without adversely affecting the overall health of the subjects or their body weight. These promising in vivo results enhance the case for dronedarone’s application in clinical settings.
Additionally, the treatment demonstrated a notable decrease in Ki67 levels, a proliferation marker that correlates with cancer aggressiveness, further validating the effectiveness of dronedarone as a therapeutic agent. The link between Ki67 reduction and diminished RB1 phosphorylation underscores the drug’s role in disrupting core cancer pathways, painting a hopeful picture for future applications in ESCC treatment protocols.
The significance of these findings cannot be overstated, especially in light of the limited options currently available for ESCC prevention and treatment. The study brings to light a potential rapid clinical application for dronedarone, which could serve as a novel adjunct therapy for patients battling this aggressive form of esophageal cancer. By leveraging an existing medication, researchers may shorten the timeline for bringing effective therapies to patients, a crucial consideration in the relentless race against cancer progression.
The research underscores not only the versatility of established drugs but also the importance of continued exploration into drug repurposing as a means to expedite the availability of effective treatments. Scientists suggest that targeting the CDK4/CDK6-RB1 axis with dronedarone could revolutionize treatment strategies for ESCC, presenting a dual benefit of maintaining treatment efficacy and minimizing side effects often associated with conventional chemotherapies.
In conclusion, this innovative approach could herald a new era for ESCC therapy, where patients benefit from existing medications that have been meticulously tested and approved for other uses. The findings pave the way for future clinical studies that entail broader applications of dronedarone in various cancer contexts, potentially transforming treatment paradigms and improving patient outcomes significantly. The compelling evidence of dronedarone’s effectiveness in ESCC continues to illuminate the path forward in oncology.
Subject of Research: Not applicable
Article Title: Dronedarone inhibits the proliferation of esophageal squamous cell carcinoma through the CDK4/CDK6-RB1 axis in vitro and in vivo
News Publication Date: 15-Oct-2024
Web References:
References:
Image Credits: Credit: Bo Li, Jing Zhang, Yin Yu, Yinhua Li, Yingying Chen, Xiaokun Zhao, Ang Li, Lili Zhao, Mingzhu Li, Zitong Wang, Xuebo Lu, Wenjie Wu, Yueteng Zhang, Zigang Dong, Kangdong Liu, Yanan Jiang
Keywords: Health and medicine