Credit: Owlstone Medical Ltd
Researchers have launched a clinical trial to develop a breath test, analysing molecules that could indicate the presence of cancer at an early stage.
This is the first test of its kind to investigate multiple cancer types.
A cancer breath test has huge potential to provide a non-invasive look into what’s happening in the body and could help to find cancer early, when treatment is more likely to be effective.
The Cancer Research UK Cambridge Centre is running the PAN Cancer trial for Early Detection of Cancer in Breath* in collaboration with Owlstone Medical** to test their Breath Biopsy® technology.
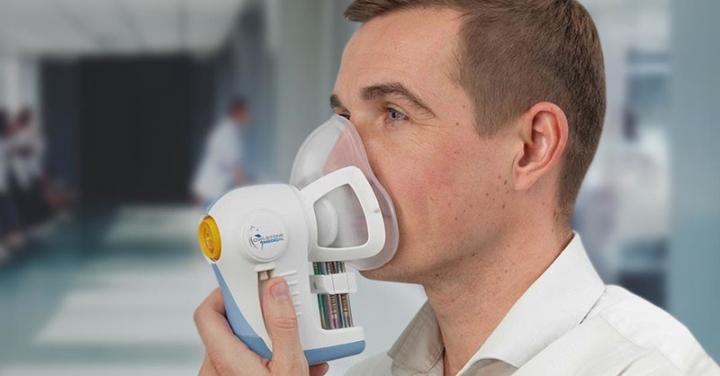
Breath samples from people will be collected in the clinical trial to see if odorous molecules called volatile organic compounds (VOCs) can be detected.
Professor Rebecca Fitzgerald, lead trial investigator at the Cancer Research UK Cambridge Centre, said: “We urgently need to develop new tools, like this breath test, which could help to detect and diagnose cancer earlier, giving patients the best chance of surviving their disease.
“Through this clinical trial we hope to find signatures in breath needed to detect cancers earlier – it’s the crucial next step in developing this technology. Owlstone Medical’s Breath Biopsy® technology is the first to test across multiple cancer types, potentially paving the way for a universal breath test.”
When cells carry out biochemical reactions as part of their metabolism they produce a range of VOCs. If their metabolism becomes altered, such as in cancer and various other conditions, cells can release a different pattern of VOCs. The researchers aim to identify these patterns using Owlstone Medical’s Breath Biopsy® technology.
The researchers in the trial will collect samples from 1,500 people, including healthy people as trial controls, to analyse VOCs in the breath to see if they can detect signals of different cancer types. The clinical trial will start with patients with suspected oesophageal and stomach cancers and then expand to prostate, kidney, bladder, liver and pancreatic cancers in the coming months.
The trial is recruiting patients to Addenbrooke’s Hospital in Cambridge who have been referred from their GP with these specific types of suspected cancer. They will be given the breath test prior to other diagnostic tests. Patients will breathe into the test for 10 minutes to collect a sample, which will then be processed in Owlstone Medical’s Breath Biopsy laboratory in Cambridge, UK.
By looking across cancer types, this trial will help unpick if cancer signals are similar or different, and how early it’s possible to pick these signals up. Some people will go on to be diagnosed with cancer, and their samples will be compared to those who don’t develop the disease.
If the technology proves to accurately identify cancer, the team hope that breath biopsies could in future be used in GP practices to determine whether to refer patients for further diagnostic tests.
Billy Boyle, co-founder and CEO at Owlstone Medical, said: “There is increasing potential for breath-based tests to aid diagnosis, sitting alongside blood and urine tests in an effort to help doctors detect and treat disease. The concept of providing a whole-body snapshot in a completely non-invasive way is very powerful and could reduce harm by sparing patients from more invasive tests that they don’t need.
“Our technology has proven to be extremely effective at detecting VOCs in the breath, and we are proud to be working with Cancer Research UK as we look to apply it towards the incredibly important area of detecting early-stage disease in a range of cancers in patients.”
Almost half of cancers are diagnosed at a late stage in England***. This highlights the importance of early detection, particularly for diseases like oesophageal cancer where only 12% of oesophageal cancer patients survive their disease for 10 years or more.
Rebecca Coldrick, 54 from Cambridge, was diagnosed in her early 30s with Barrett’s oesophagus, a condition where the cells lining the oesophagus are abnormal – often caused by acid reflux. Out of 100 people with Barrett’s oesophagus in the UK, up to 13 could go on to develop oesophageal adenocarcinoma****.
Rebecca Coldrick said: “About 20 years ago I developed acid reflux, and I began to live on Gaviscon and other indigestion remedies. I went to the doctors and shortly after I was diagnosed with Barrett’s. Every two years I have an endoscopy to monitor my condition.”
Monitoring patients to find those at high risk of developing a cancer, like oesophageal, is very intrusive for patients, who may not even develop the disease. Rebecca Coldrick decided to take part in the PAN Cancer trial for Early Detection of Cancer in Breath. A non-invasive test using this technology could help to further differentiate those likely to develop oesophageal cancer from those less likely to develop the disease.
She added: “I was very happy to take part in the trial and I want to help with research however I can. Initially, I thought I might feel a bit claustrophobic wearing the mask, but I didn’t at all. I found watching the display on the computer during the test interesting and soon we were done, without any discomfort.
“I think the more research done to monitor conditions like mine and the kinder the detection tests developed, the better.”
Dr David Crosby, head of early detection research at Cancer Research UK, said: “Technologies such as this breath test have the potential to revolutionise the way we detect and diagnose cancer in the future.
“Early detection research has faced an historic lack of funding and industry interest, and this work is a shining example of Cancer Research UK’s commitment to reverse that trend and drive vital progress in shifting cancer diagnosis towards earlier stages.”
Recognising the importance of early detection in improving cancer survival, Cancer Research UK has made research into this area one of its top priorities and will invest more than £20 million a year in early detection research by 2019.
###
For media enquiries contact Angharad Kolator Baldwin in the Cancer Research UK press office on 020 3469 8456 or, out of hours, on 07050 264 059.
For Owlstone Medical enquiries contact Sarah Jeffery at Zyme Communications on +44 (0)7771 730919 or [email protected].
Notes to editor:
*Samples will be collected by Owlstone Medical’s CE-marked ReCIVA® Breath Sampler, and then sent to the world’s first Breath Biopsy clinical laboratory for analysis at Owlstone Medical. The research is anticipated to run until 2021, collecting some 1,500 samples from clinics at Addenbrooke’s hospital in Cambridge: https:/
**While Owlstone will be funding the trial directly, none of this would be possible without the support and infrastructure provided by Cancer Research UK. The PAN Cancer trial is being conducted in collaboration with a team of leading cancer researchers at the Cancer Research UK Cambridge Centre, the University of Cambridge and Cambridge University Hospitals NHS Foundation Trust. The Chief Investigator is Professor Rebecca Fitzgerald, who is co-lead of the Cancer Research UK Cambridge Centre Early Detection Programme, Professor of Cancer Prevention at the MRC Cancer Unit, and an Honorary Consultant in Gastroenterology and General Medicine at Addenbrooke’s Hospital, Cambridge.
***For all cancers (excluding non-melanoma skin cancer) diagnosed at a late stage (3 or 4) of those with a known stage at diagnosis in England (2016). Source: http://www.
****Gatenby P, Caygill C, Wall C, et al. Lifetime risk of esophageal adenocarcinoma in patients with Barrett’s esophagus. World J Gastroenterol 2014;20(28):9611-7.
What is Breath Biopsy®?
Breath Biopsy represents an entirely new way to measure the chemical makeup of breath by measuring volatile organic compounds (VOCs), gaseous molecules that can be sampled quickly and non-invasively from breath, and enabling whole-body sampling. These compounds are produced as the end product of metabolic processes within the body, meaning that underlying changes in metabolic activity can produce particular patterns of VOCs characteristic of specific diseases.
VOCs originating from all parts of the body are captured in breath, making Breath Biopsy applicable to a wide range of diseases including cancer, inflammatory disease, infectious disease, metabolic disease, cardiovascular disease and respiratory disease. The nature of Breath Biopsy, and VOC biomarkers, make them perfectly suited to addressing two of the major challenges of healthcare today: early detection and precision medicine.
Breath collection is carried out using Owlstone Medical’s ReCIVA® Breath Sampler, which ensures reliable, reproducible collection of VOCs. Subjects breathe a controlled supply of air, and samples of their exhaled breath are captured and stabilized on Breath Biopsy Cartridges, which can then be shipped for analysis with Owlstone Medical’s Breath Biopsy analytical platform, using mass spectrometry or FAIMS to determine their VOC profile. Advanced data analytic techniques can then be applied in order to pinpoint the VOCs of interest.
About Cancer Research UK
– Cancer Research UK is the world’s leading cancer charity dedicated to saving lives through research.
– Cancer Research UK’s pioneering work into the prevention, diagnosis and treatment of cancer has helped save millions of lives.
– Cancer Research UK receives no funding from the UK government for its life-saving research. Every step it makes towards beating cancer relies on vital donations from the public.
– Cancer Research UK has been at the heart of the progress that has already seen survival in the UK double in the last 40 years.
– Today, 2 in 4 people survive their cancer for at least 10 years. Cancer Research UK’s ambition is to accelerate progress so that by 2034, 3 in 4 people will survive their cancer for at least 10 years.
– Cancer Research UK supports research into all aspects of cancer through the work of over 4,000 scientists, doctors and nurses.
– Together with its partners and supporters, Cancer Research UK’s vision is to bring forward the day when all cancers are cured.
For further information about Cancer Research UK’s work or to find out how to support the charity, please call 0300 123 1022 or visit http://www.
About Owlstone Medical http://www.
Owlstone Medical’s vision is to save 100,000 lives and $1.5 billion in healthcare costs by realising the enormous promise of breath-based diagnostics through the development and application of Breath Biopsy®. Breath Biopsy operates by detecting volatile organic compounds (VOCs) produced as the end product of metabolic processes within the body or as a result of chemicals from external sources such as diet or medication, changes in which can be characteristic of specific disease or indicate environmental exposure.
The Breath Biopsy platform includes ReCIVA®, a proprietary sample collection device that can take stable breath samples anywhere, the world’s only commercial Breath Biopsy Laboratory located in Cambridge, UK, and the development of the world’s largest Digital Breath Biobank matched to patient phenotype.
Owlstone Medical is deploying the platform to address some of the key challenges of 21st century healthcare. The focus is on the early detection of disease with an emphasis on cancer, with clinical trials underway to develop breath tests for the early detection of lung and colorectal cancer, and on precision medicine through partnerships with large pharmaceutical companies including AstraZeneca and GlaxoSmithKline to enable therapeutics to be deployed more effectively. Owlstone Medical’s technology is currently in use at over 100 sites worldwide.
Media Contact
Angharad Kolator Baldwin
[email protected]
020-346-98456




