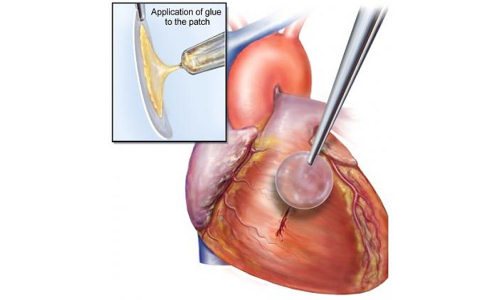
The waterproof, light-activated glue developed by researchers at Brigham and Women’s Hospital, Boston Children’s Hospital and Massachusetts Institute of Technology can successfully secure biodegradable patches to seal holes in a beating heart.
When a child is born with a heart defect such as a hole in the heart, the highly invasive therapies are challenging due to an inability to quickly and safely secure devices inside the heart.
Sutures take too much time to stitch and can cause stress on fragile heart tissue, and currently available clinical adhesives are either too toxic or tend to lose their sticking power in the presence of blood or under dynamic conditions, such as in a beating heart.
“About 40,000 babies are born with congenital heart defects in the United States annually, and those that require treatment are plagued with multiple surgeries to deliver or replace non-degradable implants that do not grow with young patients,” says Dr. Jeffrey Karp, Division of Biomedical Engineering, BWH Department of Medicine, co-senior study author of a new study that may improve how surgeons treat congenital heart defects.
The study was published on January 8, 2014, in Science Translational Medicine.
In the preclinical study, researchers from Boston Children’s Hospital, BWH and Massachusetts Institute of Technology (MIT) developed a bio-inspired adhesive that could rapidly attach biodegradable patches inside a beating heart—in the exact place where congenital holes in the heart occur, such as with ventricular heart defects.
Recognizing that many creatures in nature have secretions that are viscous and repel water, enabling them to attach under wet and dynamic conditions, the researchers developed a material with these properties that also is biodegradable, elastic and biocompatible. According to the study authors, the degradable patches secured with the glue remained attached even at increased heart rates and blood pressure.
“This adhesive platform addresses all of the drawbacks of previous systems in that it works in the presence of blood and moving structures,” says Dr. Pedro del Nido, Chief of Cardiac Surgery, Boston Children’s Hospital, co-senior study author. “It should provide the physician with a completely new, much simpler technology and a new paradigm for tissue reconstruction to improve the quality of life of patients following surgical procedures.”
Unlike current surgical adhesives, this new adhesive maintains very strong sticking power when in the presence of blood, and even in active environments.
“This study demonstrated that the adhesive was strong enough to hold tissue and patches onto the heart equivalent to suturing,” says the study’s co-first author Dr. Nora Lang, Department of Cardiac Surgery, Boston Children’s Hospital. “Also, the adhesive patch is biodegradable and biocompatible, so nothing foreign or toxic stays in the bodies of these patients.”
Importantly, its adhesive abilities are activated with ultraviolent (UV) light, providing an on-demand, anti-bleeding seal within five seconds of UV light application when applied to high-pressure large blood vessels and cardiac wall defects.
“When we attached patches coated with our adhesive to the walls of a beating heart, the patches remained despite the high pressures of blood flowing through the heart and blood vessels,” says Dr. Maria N. Pereira, Division of Biomedical Engineering, BWH Department of Medicine, co-first study author.
The researchers note that their waterproof, light-activated adhesive will be useful in reducing the invasiveness of surgical procedures, as well as operating times, in addition to improving heart surgery outcomes.
“We are delighted to see the materials we developed being extended to new applications with the potential to greatly improve human life,” said Robert Langer, ScD, MIT, study author.
The adhesive technology (and other related platforms) has been licensed to a start-up company, Gecko Biomedical, based in Paris. The company has raised 8 million Euros in their recently announced Series A financing round and expects to bring the adhesive to the market within two to three years.
Story Source:
The above story is based on materials provided by Brigham and Women’s Hospital