Nature provides mankind with a wide variety of valuable bioactive agents ranging from vitamins over vital fatty acids to cancer inhibiting substances. Many of these substances are difficult to obtain directly from the environment or can not be produced effectively by chemical total synthesis.
Scientists of the Technical University of Munich (TUM) are taking a new approach: Using synthetic biotechnology methodologies they have developed a biochemical strategy to synthesize natural and completely artificial medical agents by a templated enzyme design process. First products, a precursor of the anti-cancer medicament Taxol, anti-inflammatory substances and omega-3 fatty acids prove the successfulness of their strategy.
The bark of the pacific yew tree (Taxus brevifolia), for example, contains Taxol, an agent that is used in the treatment of breast, ovary and lung cancers. Unfortunately, this yew species is not widespread and it is protected.
Essential omega-3 fatty acids, a component of infant nutrition, for example, are currently produced predominantly from fish and crustaceans – placing a further pressure on the already strongly affected marine ecosystem.
The aim of the workgroup led by Thomas Brück, professor for Industrial Biocatalysis at the Technical University of Munich, is thus to obtain chemical substances in a sustainable manner on an industrial scale using methods of biochemistry, bioinformatics and biotechnology.
“Gold” from straw – a yeast with great potential
Now Brück and his team have succeeded in genetically altering the hitherto biotechnologically unexploited Trichosporon oleaginosus yeast by coercing it to produce essential omega 3 fatty acids alpha-linoleic acid (ALA), eicosapentaenoic acid (EPA) and the anti-inflammatory conjugated linoleic acid (CLA).
The yeast can thrive cultivation media derived from agricultural waste, including straw, wood chips, wheat bran, and even hitherto unused marine waste materials like crab shells. “This yeast is quite unique because it can also exploit monomer sugar substances, which are normally difficult to metabolize,” explains Brück. “We thus obtain valuable chemical substances from waste – without harming the environment.”
When Trichosporon oleaginosus cells become stressed in nature, from a shortage of nitrogen or phosphate, for example, they build up energy reserves in the form of fat. Even though the yeast cells no longer grow optimally, the resulting triglyceride fat reserves make up as much as 70 percent of their dry weight.
In future projects the researchers led by Brück hope to further modify the oil producing yeast so that it produces the desired fats in adequate quantities, even under normal nutrient conditions and without limiting their growth.
From simulations to custom-tailored enzymes
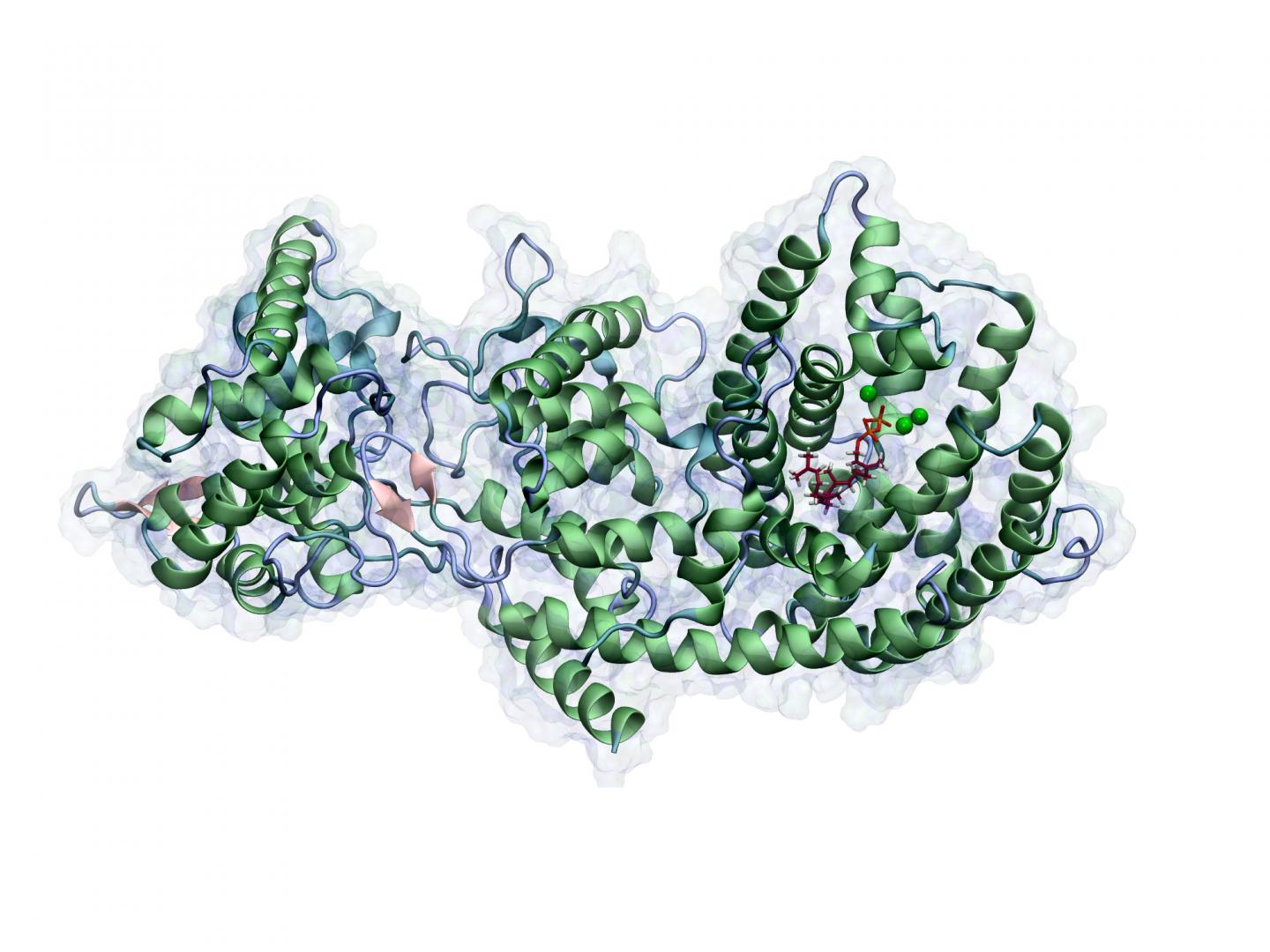
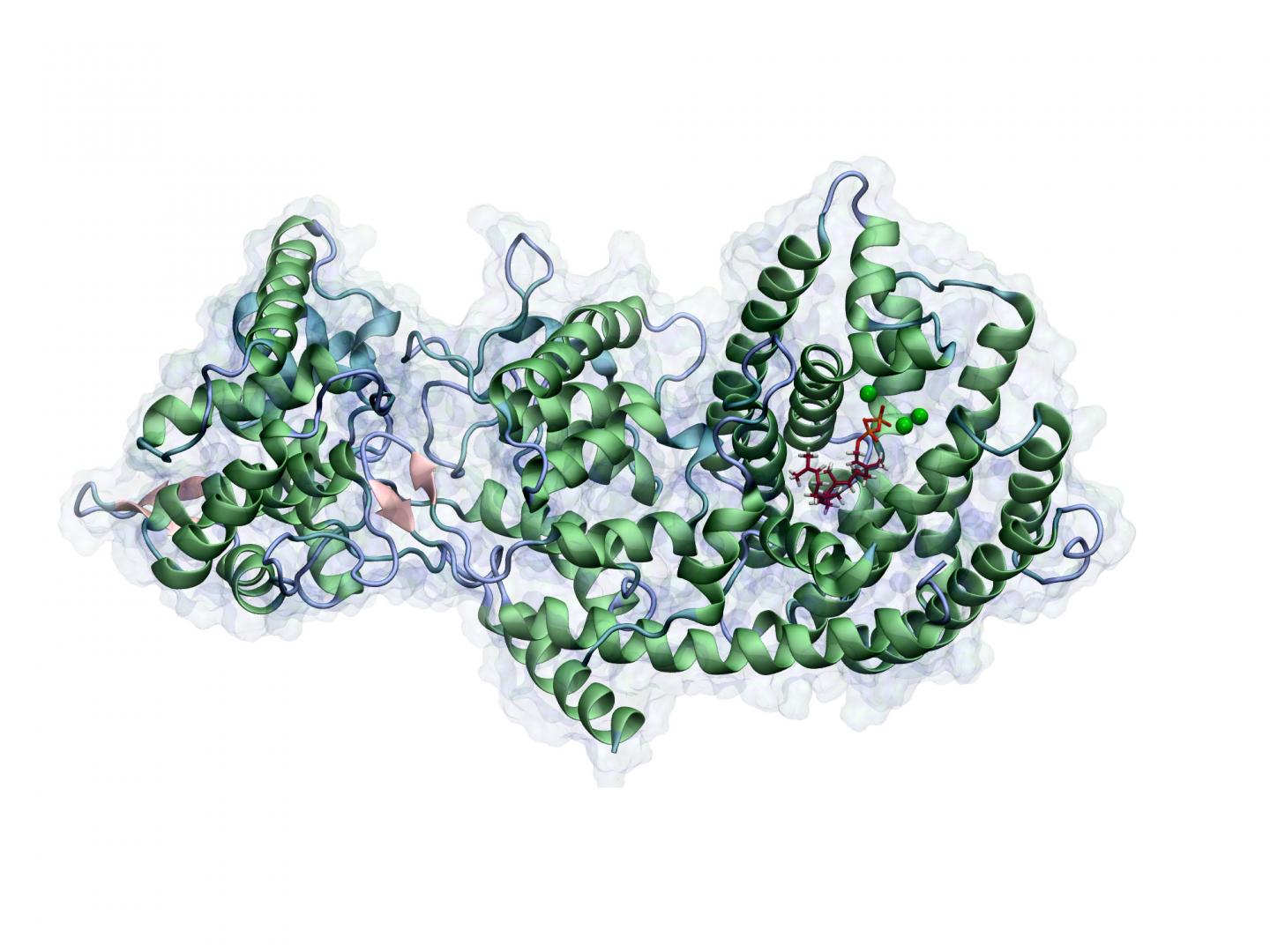
A methodology recently presented by Brück’s research group in the highly ranked scientific journal Proceedings of the National Academy of Sciences (PNAS) takes the idea one step further: Molecular-mechanical computer simulations allow them to decipher the individual steps in which a specific class of enzymes produces bioactive natural products. These include precursor stages of the cancer medication Taxol.
Employing only computer simulation, Brück and his team correctly predicted, for the first time ever, all intermediate stages in the complex cascade of reactions that take place in the presence of this enzyme. In this manner they elucidated the enzyme’s precise mode of action, as well as the relationship between its structure and function. This was previously not possible using classical biochemical methodologies.
“This approach is very promising because using computer simulation we can change enzymes in a targeted manner and predict which products will be produced as a result,” says Brück. “If we then interconnect different enzyme activities with each other, we can even create new molecules that are not found in nature.”
43-fold increase of product yield
The production of cyclooctatin, a potent anti-inflammatory agent, provides a good example: The scientists coupled a diterpene synthase enzyme with a new hydroxylase/reductase enzyme complex in an Escherichia coli-based microbial production system provided for the production of trihydroxylated diterpene cyclooctatin.
It was the in-silico discovery and experimental verification of a new reductase/ferredoxin system derived from the recently published genome of Streptomyces afghaniensis, which enabled the scientists to raise the product yield by a factor of 43, compared with the native producer.
In the future, biotechnologists could adopt approaches similar to those deployed by engineers when they develop production steps for a new automobile. Making use of the synthetic biotechnology insight, they could put together a synthesis method for new active agents using a chain of reactions with modified enzymes. This would significantly reduce the long and arduous process of “puzzling out” new synthesis routes in the laboratory, as commonly applied today.
The present research of Brück’s research group was funded by the European Community (ChiBio project), the German Federal Ministry of Education and Research (Advanced Biomass Value, SysBio Terp, OMCBP) and the German Federal Ministry of Economics (Bio@Jet project), as well as the Bavarian Ministry for Science (Algenflugkraft), the Bavarian Ministry of Economics (Algenflugkraft and sustainable production of bioinsecticides) and the Bavarian Ministry of the Environment (geo-biotechnology and PHB).
Due to the enormous potential of these methodologies, the Technical University of Munich created a new teaching and research focus, Synthetic Biotechnology, in May. The Werner Siemens Foundation is funding the setup of the new research focus to the tune of 11.5 million Euro.
###
Publications:
P. Schrepfer, A. Buettner, C. Goerner, M. Hertel, J. van Rijn, F. Wallrapp, W. Eisenreich, V. Sieber, R. Kourist, T. Brück; Identification of amino acid networks governing catalysis in the closed complex of class I terpene synthases; PNAS, 2016, 113(8), E958-E967 – DOI: 10.1073/pnas.1519680113
Görner, C., Redai, V., Bracharz, F., Schrepfer, P., Garbe, D., & Brück, T.; Genetic engineering and production of modified fatty acids by the non-conventional oleaginous yeast Trichosporon oleaginosus ATCC 20509. Green Chemistry, 2016, 18, 2037-2046 – DOI: 10.1039/c5gc01767j
Christian Görner, Patrick Schnepfer, Veronika Redai, Frank Wallrapp, Bernhard Loll, Wolfgang Eisenreich, Martin Haslbeck and Thomas Brück; Identification, characterization and molecular adaptation of class I redox systems for the production of hydroxylated diterpenoids; Micobial Cell Factories (2016) 15:86 – DOI: 10.1186/s12934-016-0487-6
Media Contact
Dr. Andreas Battenberg
[email protected]
49-892-891-0510
@TU_Muenchen
http://www.tum.de
The post A new molecular toolkit for the de-novo design of bioactive agents appeared first on Scienmag.