Circularly conjugated compounds with 4n+2 pi-electrons are known as aromatic compounds. They are generally stable and are therefore found in our surroundings. On the other hand, anti-aromatic compounds with 4n pi-electrons have been conventionally considered unstable, and the creation of stable anti-aromatic compounds has been one of the challenging issues in organic chemistry. Several studies on the synthesis, isolation, and characterization of stable and clearly anti-aromatic compounds have been reported in recent years. In general, anti-aromatic compounds are considered to be more susceptible to substituents than aromatic compounds because of their narrower HOMO-LUMO gap. However, there has been no systematic study of such substituent effects in anti-aromatic compounds.
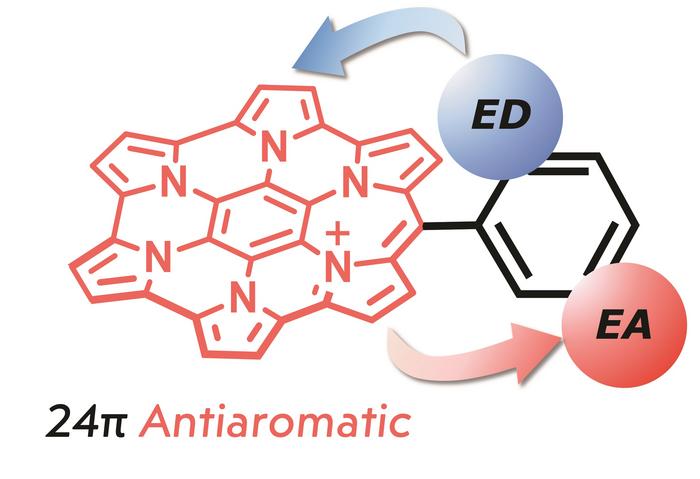
This research group has been conducting studies on the synthesis and properties of hexapyrrolohexaazacoronene (HPHAC), a nitrogen-containing polycyclic aromatic compound consisting of pyrrole. Furthermore, homoHPHAC, a pi-extended analog of HPHAC, was reported to show global anti-aromaticity as a monocation and global aromaticity as a trication. In this study, a new synthetic method for homoHPHACs using Friedel-Crafts-type intramolecular condensation reactions was developed, and a series of compounds with electron-donating to electron-accepting substituents were synthesized. The effects of substituents on structural, optical, redox, and antiaromatic (aromatic) properties were demonstrated. In conjunction with computational chemistry, it was shown that both anti-aromatic (monocation) and aromatic (tricationic) properties were the strongest in compounds with electron-accepting substituents.
Various approaches to the use of organic compounds as electronic materials are being investigated from the viewpoints of reducing environmental impact and providing versatility in functional control. The attempt to control electronic properties by introducing substituents into anti-aromatic compounds is expected to provide new design guidelines for molecular materials.
Credit: Masayoshi Takase(Ehime University)
Circularly conjugated compounds with 4n+2 pi-electrons are known as aromatic compounds. They are generally stable and are therefore found in our surroundings. On the other hand, anti-aromatic compounds with 4n pi-electrons have been conventionally considered unstable, and the creation of stable anti-aromatic compounds has been one of the challenging issues in organic chemistry. Several studies on the synthesis, isolation, and characterization of stable and clearly anti-aromatic compounds have been reported in recent years. In general, anti-aromatic compounds are considered to be more susceptible to substituents than aromatic compounds because of their narrower HOMO-LUMO gap. However, there has been no systematic study of such substituent effects in anti-aromatic compounds.
This research group has been conducting studies on the synthesis and properties of hexapyrrolohexaazacoronene (HPHAC), a nitrogen-containing polycyclic aromatic compound consisting of pyrrole. Furthermore, homoHPHAC, a pi-extended analog of HPHAC, was reported to show global anti-aromaticity as a monocation and global aromaticity as a trication. In this study, a new synthetic method for homoHPHACs using Friedel-Crafts-type intramolecular condensation reactions was developed, and a series of compounds with electron-donating to electron-accepting substituents were synthesized. The effects of substituents on structural, optical, redox, and antiaromatic (aromatic) properties were demonstrated. In conjunction with computational chemistry, it was shown that both anti-aromatic (monocation) and aromatic (tricationic) properties were the strongest in compounds with electron-accepting substituents.
Various approaches to the use of organic compounds as electronic materials are being investigated from the viewpoints of reducing environmental impact and providing versatility in functional control. The attempt to control electronic properties by introducing substituents into anti-aromatic compounds is expected to provide new design guidelines for molecular materials.
Journal
Chemical Science
DOI
10.1039/D2SC07037E
Article Publication Date
6-Jun-2023




