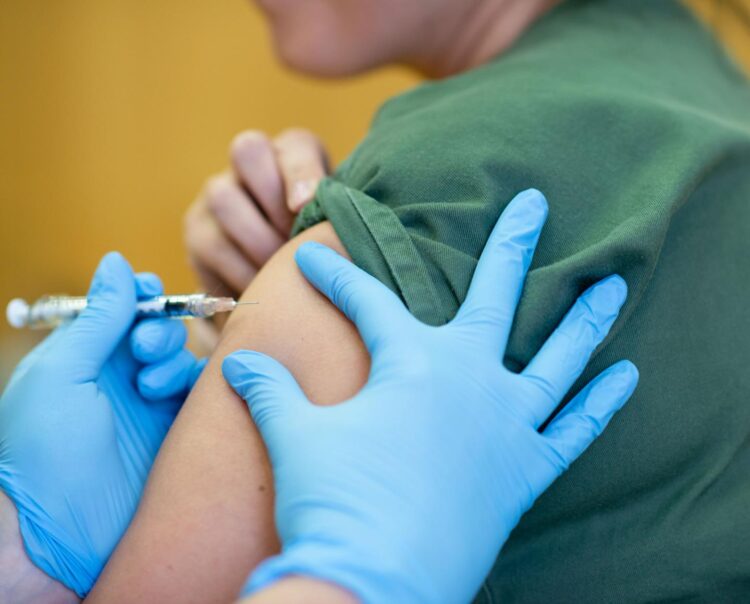
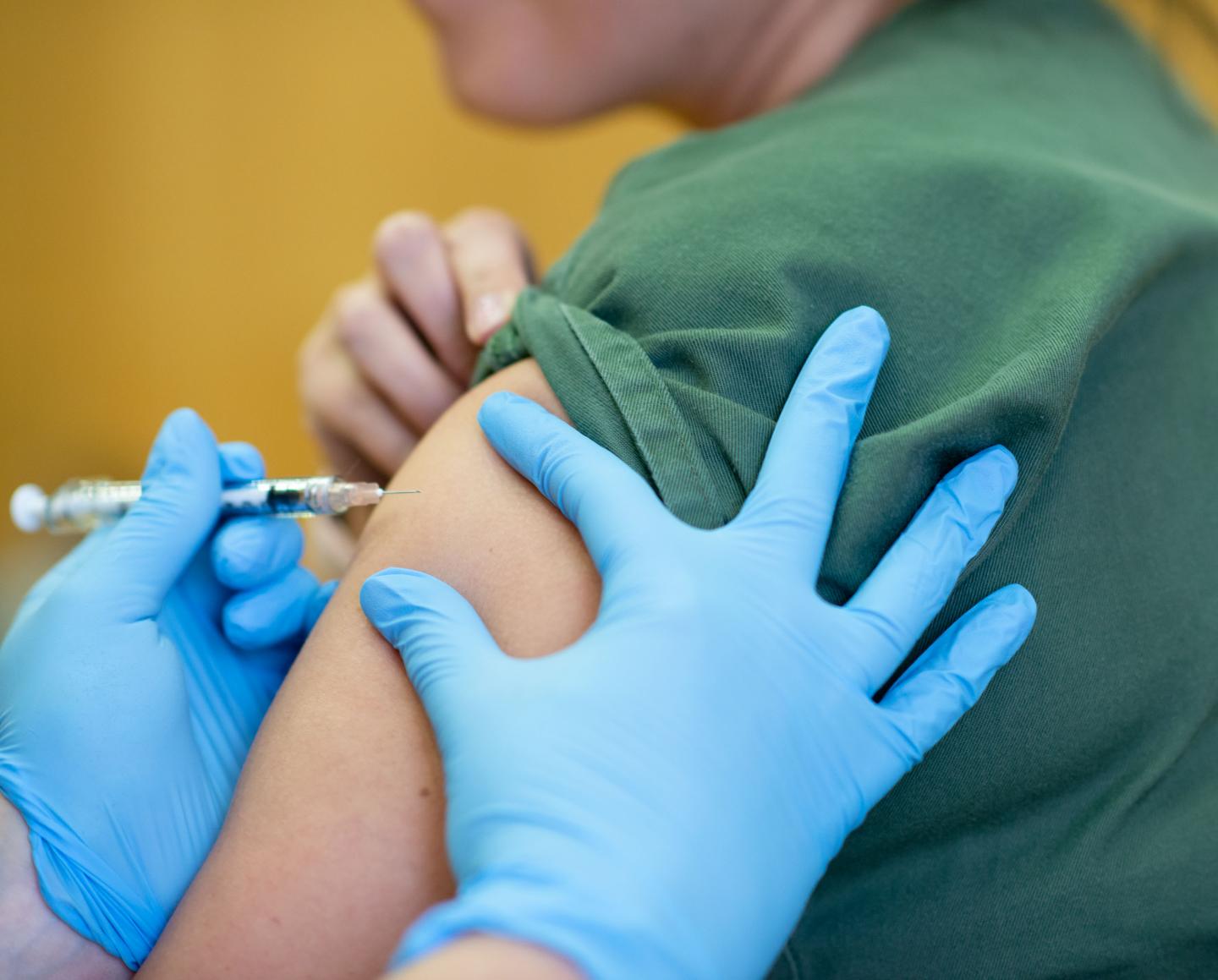
An $10 million grant from the Bill & Melinda Gates Foundation will allow the Murdoch Children’s Research Institute’s (MCRI) clinical trial of the BCG vaccine against COVID-19 to extend to 10,000 healthcare workers across Australia and Europe
Credit: MCRI
An A$10 million grant from the Bill & Melinda Gates Foundation will allow the Murdoch Children’s Research Institute’s (MCRI) clinical trial of the BCG vaccine against COVID-19 to extend to 10,000 healthcare workers across Australia, Spain and The Netherlands.
The grant will allow the MCRI team to expand the BRACE trial from the original target of 4000 healthcare workers.
In The Netherlands and Spain, the Radboud University Medical Center and UMC Utrecht will begin enrolling 4000 healthcare workers across 13 sites in the coming weeks.
The BRACE trial, which commenced on March 27, has already enrolled more than 2500 workers in hospitals across Australia.
The multi-centre randomised controlled trial, which started at The Royal Children’s Hospital, will see half of healthcare workers given the BCG vaccine to test whether it can protect those exposed to SARS-CoV-2 from developing severe symptoms by boosting their ‘frontline’ immunity.
BCG was originally developed against tuberculosis, and is still given to over 130 million babies worldwide each year for that purpose. The BRACE trial builds on previous research which showed that BCG can provide some protection against respiratory viral infections and a study in which BCG reduced virus levels and enhanced immunity to a virus with a structure of a similar type to SARS-CoV-2.
Professor Nigel Curtis, who leads the BRACE trial, thanked the Bill & Melinda Gates Foundation for generously supporting this research effort. Professor Curtis is a clinician-scientist who heads MCRI’s Infectious Diseases Research Group, Professor of Paediatric Infectious Diseases at The University of Melbourne and Head of Infectious Diseases at The Royal Children’s Hospital Melbourne.
“This funding will be crucial to quickly enable us to expand the BRACE trial to Sydney in Australia and The Netherlands and Spain internationally,” he said.
“It will be imperative to help our researchers show whether BCG vaccination improves ‘innate’ immunity in frontline healthcare workers to buy crucial time to develop and importantly, validate, a specific anti-COVID-19 vaccine.”
Philanthropic support has allowed the trial’s rapid development and rollout in Australia. This support has included A$700,000 from Sarah and Lachlan Murdoch, A$400,000 from The Royal Children’s Hospital Foundation (RCHF), A$1.5M from The Minderoo Foundation and A$200,000 from the South Australian government.
Following the Melbourne launch of the trial in March, additional sites in Melbourne, Adelaide and Perth were announced in mid-April.
Professor Curtis said, “We have dealt with the pandemic extremely well in Australia with rapid and thorough physical distancing, contact tracing and quarantine where appropriate.
This new funding from the Gates Foundation allows other countries to also test whether additional preventative measures may help protect healthcare workers.
“These sorts of trials normally take around eight to 12 months to start, but with the early support of philanthropy, we were able to start in record time within three weeks.
“Since beginning the BRACE trial we have been inundated with requests from other hospitals wanting to be involved, both in Australia and internationally. This funding will allow us to begin delivering on those requests.”
Professor Marc Bonten from UMC Utrecht said, “We are looking forward to collaborating with Professor Curtis and his team. We are very pleased to now be joining this international trial to see if BCG can help lessen COVID-19 symptoms in Dutch healthcare workers.”
Professor Mihai Netea echoed those sentiments saying, “At Radboud UMC we have spent more than 10 years investigating BCG’s off-target effects, including previous research with Professor Curtis. In a time of crisis such as this, we thank the Bill & Melinda Gates Foundation for their agile and generous contribution.”
Professors Curtis and Netea, along with WHO Director-General Dr Tedros Ghebreyesus, recently emphasised in a letter to The Lancet the need for BCG vaccine to only be given in the context of clinical trials when investigating its off-target effects.
BCG vaccines are already in short supply, and indiscriminate use could jeopardise the supply needed to protect children against tuberculosis in high-risk areas.
In Australia, the South Australian site is coordinated through the South Australian Health and Medical Research Institute, and was enabled by a A$200,000 funding announcement from the South Australian state government.
Three sites in Perth are coordinated by the Telethon Kids Institute, and funded by the Minderoo Foundation which contributed A$1.5 million.
The additional sites in Melbourne are at Monash Health and Epworth Health, and The Children’s Hospital at Westmead in Sydney.
MCRI Director, Professor Kathryn North, said, “The philanthropic leadership shown by our valued donors is vital to support urgent medical research at this critical time.”
“Australia has proven itself to be a global leader in ‘flattening the curve’ and we are pleased to now be in a position to help the rest of the world. We are still in a race against time.”
Visit the MCRI website for more information on the BRACE trial.
###
Media Contact
Thomas Keeble
[email protected]