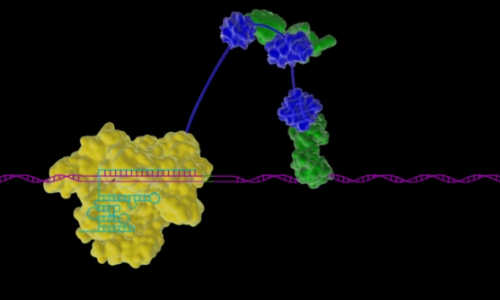
When it comes to gene expression – the process by which our DNA provides the recipe used to direct the synthesis of proteins and other molecules that we need for development and survival – scientists have so far studied one single gene at a time. A new approach developed by Harvard geneticist George Church, Ph.D., can help uncover how tandem gene circuits dictate life processes, such as the healthy development of tissue or the triggering of a particular disease, and can also be used for directing precision stem cell differentiation for regenerative medicine and growing organ transplants.
In this technical animation, Wyss Institute researchers instruct how they engineered a Cas9 protein to create a powerful and robust tool for activating gene expression. The novel method enables Cas9 to switch a gene from off to on and has the potential to precisely induce on-command expression of any of the countless genes in the genomes of yeast, flies, mice, or humans. Credit: Wyss Institute at Harvard University
The findings, reported by Church and his team of researchers at the Wyss Institute for Biologically Inspired Engineering at Harvard University and Harvard Medical School in Nature Methods, show promise that precision gene therapies could be developed to prevent and treat disease on a highly customizable, personalized level, which is crucial given the fact that diseases develop among diverse pathways among genetically-varied individuals. Wyss Core Faculty member Jim Collins, Ph.D., was also a co-author on the paper. Collins is also the Henri Termeer Professor of Medical Engineering & Science and Professor in the Department of Biological Engineering at the Massachusetts Institute of Technology.
The approach leverages the Cas9 protein, which has already been employed as a Swiss Army knife for genome engineering, in a novel way. The Cas9 protein can be programmed to bind and cleave any desired section of DNA – but now Church’s new approach activates the genes Cas9 binds to rather than cleaving them, triggering them to activate transcription to express or repress desired genetic traits. And by engineering the Cas9 to be fused to a triple-pronged transcription factor, Church and his team can robustly manipulate single or multiple genes to control gene expression.
“In terms of genetic engineering, the more knobs you can twist to exert control over the expression of genetic traits, the better,” said Church, a Wyss Core Faculty member who is also Professor of Genetics at Harvard Medical School and Professor of Health Sciences and Technology at Harvard and MIT. “This new work represents a major, entirely new class of knobs that we could use to control multiple genes and therefore influence whether or not specific genetics traits are expressed and to what extent – we could essentially dial gene expression up or down with great precision.”
Such a capability could lead to gene therapies that would mitigate age-related degeneration and the onset of disease; in the study, Church and his team demonstrated the ability to manipulate gene expression in yeast, flies, mouse and human cell cultures.
“We envision using this approach to investigate and create comprehensive libraries that document which gene circuits control a wide range of gene expression,” said one of the study’s lead authors Alejandro Chavez, Ph.D., Postdoctoral Fellow at the Wyss Institute. Jonathan Schieman, Ph.D, of the Wyss Institute and Harvard Medical School, and Suhani Vora, of the Wyss Institute, Massachusetts Institute of Technology, and Harvard Medical School, are also lead co-authors on the study.
The new Cas9 approach could also potentially target and activate sections of the genome made up of genes that are not directly responsible for transcription, and which previously were poorly understood. These sections, which comprise up to 90% of the genome in humans, have previously been considered to be useless DNA “dark matter” by geneticists. In contrast to translated DNA, which contains recipes of genetic information used to express traits, this DNA dark matter contains transcribed genes which act in mysterious ways, with several of these genes often having influence in tandem.
But now, that DNA dark matter could be accessed using Cas9, allowing scientists to document which non-translated genes can be activated in tandem to influence gene expression. Furthermore, these non-translated genes could also be turned into a docking station of sorts. By using Cas9 to target and bind gene circuits to these sections, scientists could introduce synthetic loops of genes to a genome, therefore triggering entirely new or altered gene expressions.
The ability to manipulate multiple genes in tandem so precisely also has big implications for advancing stem cell engineering for development of transplant organs and regenerative therapies.
“In order to grow organs from stem cells, our understanding of developmental biology needs to increase rapidly,” said Church. “This multivariate approach allows us to quickly churn through and analyze large numbers of gene combinations to identify developmental pathways much faster than has been previously capable.”
To demonstrate this point, the researchers used it to grow brain neuron cells from stem cells and found that using the approach to program development of neuronal cells was 40-fold more successful than prior established methods. This is the first time that Cas9 has been leveraged to efficiently differentiate stem cells into brain cells.
The new approach is also compatible to be used in combination with other gene editing technologies. Church and his team have previously made breakthroughs by developing a gene editing mechanism for therapeutic applications and gene drives for altering traits in plant and animal species.
“This newest tool in the Cas9 genome engineering arsenal offers a powerful new way to control cell and tissue function that could revolutionize virtually all areas of science and medicine, ranging from gene therapy to regenerative medicine and anti-aging,” said Wyss Institute Founding Director Donald Ingber, M.D., Ph.D, who is also the Judah Folkman Professor of Vascular Biology at Harvard Medical School and Boston Children’s Hospital and Professor of Bioengineering at Harvard SEAS.
Story Source:
The above story is based on materials provided by University of Wyss Institute for Biologically Inspired Engineering.