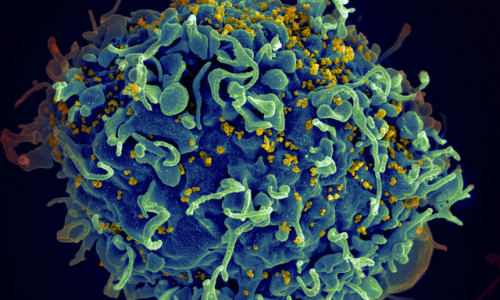
Normal microorganisms in the intestines appear to play a pivotal role in how the HIV virus foils a successful attack from the body’s immune system, according to new research from Duke Medicine.
The study, published Aug. 13, 2014, in the journal Cell Host & Microbe, builds on previous work from researchers at the Duke Human Vaccine Institute that outlined a perplexing quality about HIV: The antibodies that originally arise to fight the virus are ineffective.
These initial, ineffective antibodies target regions of the virus’s outer envelope called gp41 that quickly mutates, and the virus escapes being neutralized. It turns out that the virus has an accomplice in this feat – the natural microbiome in the gut.
“Gut flora keeps us all healthy by helping the immune system develop, and by stimulating a group of immune cells that keep bacteria in check,” said senior author Barton F. Haynes, M.D., director of the Duke Human Vaccine Institute. “But this research shows that antibodies that react to bacteria also cross-react to the HIV envelope.”
Haynes said the body fights most new infections by deploying what are known as naïve B cells, which then imprint a memory of the pathogen so the next time it encounters the bug, it knows how to fight it.
But when the HIV virus invades and begins replicating in the gastrointestinal tract, no such naïve B cells are dispatched. Instead, a large, pre-existing pool of memory B cells respond – the same memory B cells in the gut that fight bacterial infections such as E coli.
This occurs because the region of the HIV virus that the immune system targets, the gp41 region on the virus’s outer envelope, appears to be a molecular mimic of bacterial antigens that B cells are primed to target.
“The B cells see the virus and take off – they make all these antibodies, but they aren’t protective, because they are targeted to non-protective regions of the virus envelope.”
Haynes and colleagues said the findings were confirmed in tests of people who were not infected with HIV. Among non-infected people, the researchers isolated mutated gp41-gut flora antibodies that cross-react with intestinal bacteria.
“The hypothesis now is that the gp41 antibody response in HIV infection can be derived from a pre-infection memory B cell pool triggered by gut bacteria that cross-reacts with the HIV envelope,” said lead author Ashley M. Trama. “This supports the notion that the dominant HIV antibody response is influenced by previously activated memory B cells that are present before HIV infection and are cross-reactive with intestinal bacteria.”
Haynes said the finding provides compelling new information for HIV vaccine development, which is the next phase of research.
“Not only can gut flora influence the development and function of the immune system, but perhaps also pre-determine our reaction to certain infections such as HIV,” Haynes said.
Story Source:
The above story is based on materials provided by Duke Medicine.