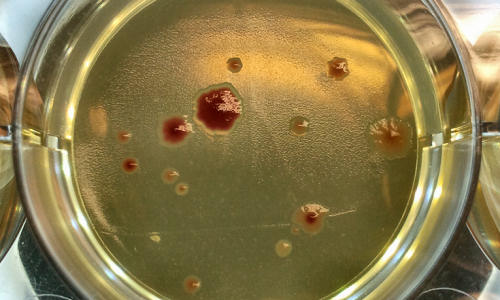
Scientists from the University of Manchester have discovered that microbes in smaller groups are more likely to mutate, resulting in higher rates of antibiotic resistance.
Photo Credit: Rok Krašovec
The study, published today in ‘Nature Communications’ and jointly funded by the Wellcome Trust and the Engineering and Physical Sciences Research Council, explored mutation rates in E. coli bacteria.
The researchers found out that the rate of mutation varied according to how many of the bacteria there were. Surprisingly, they discovered that larger groups of bacteria were less likely to mutate than smaller groups.
The more ‘lonely’ bacteria mutated more, and developed greater resistance to the well-known antibiotic Rifampicin, used to treat tuberculosis.
Dr Chris Knight, joint lead author on the study with Dr Rok Krašovec, from the University of Manchester, said: “What we were looking for was a connection between the environment and the ability of bacteria to develop the resistance to antibiotics. We discovered that the rate at which E. coli mutates depends upon how many ‘friends’ it has around. It seems that more lonely organisms are more likely to mutate.”
This change of the mutation rate is controlled by a form of social communication known as quorum sensing – this is the way bacteria let each other know how much of a crowd there is. Quorum sensing involves the release of signalling molecules by bacteria: the denser the population, the more of these molecules released and detected. This helps the bacteria understand their environment and coordinate their behaviour to improve their defence mechanisms and adapt to the availability of nutrients.
Dr Krašovec said: “We were able to change their mutation rates by changing who they shared a test-tube with, which could mean that bacteria manipulate each other’s mutation rates. It also suggests that mutation rates could be affected when bacteria are put at low densities, for instance by a person taking antibiotics.”
The rate of mutation was found to depend on the gene luxS, which is known to be involved in quorum sensing in a wide range of bacteria.
The team now hopes to find ways to control this signalling for medical applications in a future study funded by the Biotechnology and Biological Sciences Research Council.
“Eventually this might lead to interventions to control mutation rates, for instance to minimise the evolution of antibiotic resistance, allowing antibiotics to work better,” said Dr Knight.
Dr Mike Turner, Head of Infection and Immunobiology at the Wellcome Trust, said: “Antibiotic resistance is a real threat to disease control and public health today. Any insight into the origins of such resistance is valuable in the fight to prevent it. Chris Knight and his team have gained a fundamental understanding of bacterial communication and the development of mutations which in the long run could contribute to more potent antibiotics and better control of bacterial disease”.
Story Source:
The above story is based on materials provided by Wellcome Trust, Kath Paddison.