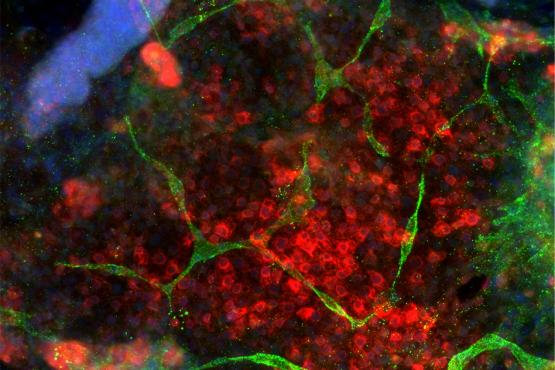
Under the microscope, they look like art: a red dapple with green crescents, deep blue and purple spots, angular green dabs. But these are actually cells, highlighted with fluorescent dyes and antibodies, that MIT senior Nathan Kipniss grows and studies.
Nathan Kipniss in his lab – Photo Credits: Allegra Boverman
Kipniss — a biological engineering major — does synthetic biology research in the laboratory of Ron Weiss, an associate professor of computer science and biological engineering. There, he and other researchers manipulate genetic code to “program” stem cells in order to create more complex structures, such as liver and pancreatic tissues.
Also a cellist in the MIT Symphony Orchestra (MITSO), and former house chair of Simmons Hall, Kipniss grew up in Schenectady, N.Y., with his mother and twin brother. His mother, a nurse, fostered Kipniss’ early interest in science. When he was 8, she gave him a microscope kit complete with glass slides of real human tissues, carefully prepared by a histologist in the hospital where she worked. Peering through the lens, Kipniss was in awe. “It was fascinating to see tissues from the body in such detail,” Kipniss recalls.
Now, Kipniss creates his own slides: microscopic snapshots of tissues that he grows in the lab.
Complex tissues from simple cells
A tissue is made up of many different types of cells, each of which plays its own role in the tissue’s function. In pancreatic tissue, beta cells regulate the body’s blood sugar by secreting insulin when it is needed. In Weiss’ lab, Kipniss collaborates with postdoc Patrick Guye in attempting to produce functional beta cells from human-induced pluripotent stem cells.
“It’s been notoriously difficult to generate beta cells that function properly,” Kipniss says. “For example, the cells might be able to produce insulin but not secrete it, or they can secrete it but not in the right doses.” If functioning beta cells could be made in a lab, especially in the context of other supportive cell types, Kipniss adds, they might eventually be able to help people with diabetes whose own beta cells are not working correctly.
In most efforts to create specific types of cells, researchers have started with a plate of stem cells and added molecules that induce changes in the cells and cause them to differentiate. However, this typically results in a homogenous population of cells that lacks proper function. Weiss’ research group is trying a novel approach: using genetic circuits to “program” stem cells to develop into the elusive beta cells.
The concept of a genetic circuit might sound peculiar: Can a cell be programmed, like a computer? But cells and computers are actually quite similar, Kipniss says, in that both involve an input, some sort of information processing, and an output. In a computer, you enter a certain command, and the machine executes that command. In a cell, you can do the same thing: For example, you could create and insert DNA that tells a stem cell to turn a particular color once it reaches a given cell type. In this way, a cell can be programmed, but by using segments of genetic information instead of typed words.
Liver cells and vasculature Image: Nathan Kipniss/Weiss Lab
Previous research has provided biologists with a large array of functions, such as enzymes to snip DNA segments in particular places, and other enzymes to stitch them back together. When Kipniss wants to create a new program for the cells, he reads up on previous work so he can make an educated guess about the combination and temporal sequence of genetic components — namely, genes and promoters — that will make the cell do what he wants. After he has created the genetic program, he uses a virus to deliver the DNA to the cells and integrate it into the genome.
This method has some key advantages. “With genetic circuits, you can get control at the single-cell level,” Kipniss says — that is, researchers can program multiple stem cell clusters in different ways, and then combine them. Each of the cells would develop as they had been programmed, potentially into the different cell types necessary for a complete tissue.
Between the project to create pancreatic beta cells and a second project on cell signaling in complex liver tissue formation, Kipniss is in the lab daily — whether constructing DNA, caring for the cells, or running various experiments to probe cellular behavior and tissue organization. “But I don’t realize how much time everything takes,” Kipniss says. “The problems are so interesting; biology can get wonderfully complex very quickly. And I’m working with really awesome people.”
Parts of a whole
Outside of the lab, Kipniss enjoys campus life as part of MITSO and his dormitory, Simmons Hall.
Kipniss began playing cello in elementary school; at MIT, he has relished the challenge of playing in an orchestra. “Coming to MIT, I was glad to see the presence of the arts on campus,” he says.
He noted that while performing in a symphony, “Every musician in a section has to blend their sound to contribute to the overall piece, but you also have to listen for people in other parts of the orchestra, to anticipate what’s going to happen. You have to have an intuition about it.”
One of Kipniss’ favorite pieces is a ballet score by Igor Stravinsky called “The Rite of Spring,” which MITSO performed last year. “It’s a crazy piece,” he says. “Parts of the score get incredibly chaotic, with instruments playing distinct rhythms against each other and clashing keys to create dissonance.”
It is the complexity of the piece that Kipniss loves. “It also represents what I love about people here — many have these talents outside of science and engineering,” he says. “The caliber of musicians that you find here is just really incredible.”
As the house chair of Simmons, Kipniss learned to manage a different sort of orchestra: the cacophony of voices at meetings for the dorm, which houses nearly 350 undergraduates. “By having an active dorm government, we’ve been able to do some really fun and cool things,” Kipniss says. “Simmons is turning into an ever more vibrant community where everyone has diverse interests.”
After he graduates in June, Kipniss looks forward to graduate school and further research on stem cells and tissue engineering. “I’m really interested in embracing complexity in engineering,” Kipniss says. “Instead of reducing the problem, let’s examine biological complexity, learn from it, and see how we can build with it to eventually solve problems related to medicine.”
Story Source:
The above story is based on materials provided by MIT News Office, Jessica Fujimori.