Credit: POSTECH
Cerebral aneurysms are malformations caused by abnormalities on the walls of blood vessels in the brain. When these blood vessels rupture, about 30% of the sufferers die on the spot, giving these the rightful label of ‘ticking bombs in the head’. Recently, a research team at POSTECH has come up with a new treatment that can disassemble these time bombs by filling the aneurysm in blood with a new method.
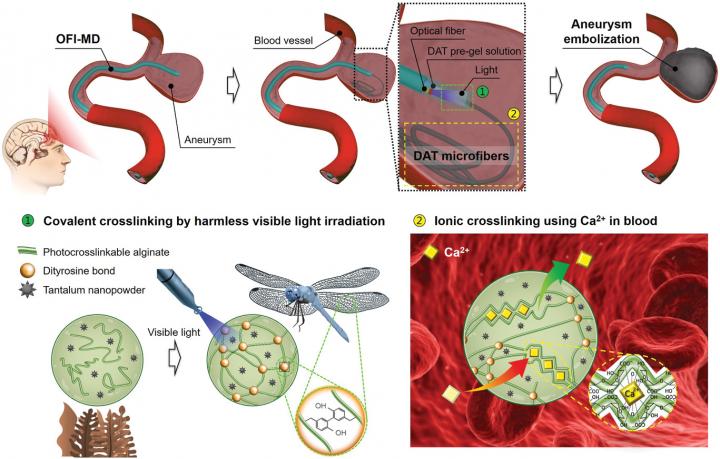
In order to overcome the shortcomings of coil embolization, POSTECH’s joint research team (Professor Joonwon Kim and Dr. Jongkyeong Lim of the Department of Mechanical Engineering with Professor Hyung Joon Cha and Ph.D. candidate Geunho Choi of the Department of Chemical Engineering) has developed a new structurally stable, biocompatible embolization material that does not decompose in the human body. In addition, a new concept of cerebral aneurysm therapy (treatment device) capable of stably forming and controlling the material in the form of microfibers in the intravascular environment was presented. These research findings were published as the outside back cover paper of Advanced Materials on April 8, 2021.
Early detection of cerebral aneurysms is increasing due to periodic health examinations. The current treatment method uses coil embolization, which lowers the direction and pressure of internal blood flow by filling the aneurysm with a platinum coil before it ruptures. However, coil embolization surgery is a financial burden since it requires many platinum coils (KRW 600,000 (USD 550) per coil) commensurate to the aneurysm size. Moreover, due to the coil’s fine spring structure, the aneurysm may burst during surgery or the inside of the aneurysm may not be completely filled. There are also other problems in which the coil may be detached from the affected area due to recompression caused by the low filling rate after operation.
Hydrogels, which respond to various stimuli such as temperature, pH, and light, and exhibit soft mechanical strength, have attracted great attention as an embolic material that can fill an aneurysm at a high rate. Among them, photocrosslinkable hydrogels are considered to be most suitable for embolization thanks to their easy spatiotemporal control. But their practical usage is limited. In addition, materials being developed for aneurysm embolization currently use high concentrations of biologically inactive synthetic hydrogels that are toxic and not biocompatible. These can also cause severe swelling in the body – often leading to ruptures – and have thwarted their commercialization. In addition, conventional surgical methods could not be applied to clinical applications because it was impossible to produce and control hydrogels using light in an intravascular environment, which has a tortuous geometric structure and high absorbance.
The research team led by Professor Hyung Joon Cha of the Department of Chemical Engineering proposed an alginate-based hydrogel derived from algae that can be double-crosslinked as a new material for aneurysm embolization. This new embolic material exhibits excellent biocompatibility and uses the synergistic effect of rapid covalent crosslinking by irradiation with the harmless visible light and ionic crosslinking using calcium ions present in the blood. In addition, since no degrading enzymes exist in the human body, it does not degrade and exhibits outstanding structural stability without swelling. This can safely and effectively fill the aneurysm and successfully prevent rupture. Since it is possible to mount a contrast medium, it exhibits radiopacity so that the embolic material applied through CT or MRI can be monitored continuously for a long time.
The research team led by Professor Joonwon Kim of the Department of Mechanical Engineering developed a microfluidic device with an integrated optical fiber. This device is a new concept surgical device that can stably produce and control photocrosslinkable hydrogels in the form of microfibers in an extreme intravascular environment, which has a tortuous geometric structure and high absorbance.
Double-crosslinked alginate-based hydrogel microfibers produced and controlled in these microfluidic devices can safely and evenly fill the aneurysms. During this stage, the microfibers intertwine with each other to form a lump to block the flow of fluid from entering the aneurysm and maintain the structural shape and constant mechanical strength without dissociation even in the pulsating environment after surgery. This minimizes a resurge of the pressure inside the aneurysm or its rupture.
“This research is the first in the world to develop a new material for embolization that may be biocompatible without side effects and be stably maintained in the human body for a long time,” remarked Professor Hyung Joon Cha. “We plan to deliberate on commercialization through a technology transfer.”
Professor Joonwon Kim explained, “This research is the first in the world to develop a method that can be used to treat aneurysms by microfibrillating a photocrosslinkable hydrogel microfiber in blood vessels.” He added, “It is anticipated these materials will be effectively applicable to many vascular diseases requiring embolization.”
“The new concept of embolization method developed in this study was verified using the Advanced Dynamic Angio Model (ADAM) simulator implemented by the new deep coating technology and 3D vascular replica production technology using liquid embolic materials previously developed by the research team. The ADAM simulator is a system that provides a virtual environment that is very similar to the surgical environment of a real patient, and can be used for simulation of various vascular diseases,” explained Professor Kim.
###
This research was conducted with the support from the research-centered hospital nurturing R&D project supported by the Ministry of Health and Welfare and the Korea Health Industry Development Institute, and the Mid-career Research Program and the Nano-New Materials Core Technology Development Program funded by the Ministry of Science and ICT and the National Research Foundation of Korea.
Media Contact
Jinyoung Huh
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




