Experiments with a novel time-lapse system show that potassium channel exhibit hysteresis in their responses to membrane tension
Credit: Masayuki Iwamoto from University of Fukui
Ion channels play an indispensable role in cellular physiology, and understanding the physical features that affect ion channel functions is a matter of considerable interest to biologists. Given that mechanosensitivity is an intrinsic feature of cells, the complex set of mechanical stresses acting on a cell at any time represents an important consideration in the field of cellular physiology. In fact, stretching forces created by mechanical stress are sometimes necessary to activate ion channels. As Professor Masayuki Iwamoto and Professor Shigetoshi Oiki of the University of Fukui explain, “Mechanical stresses change the level of cell membrane tension, and stretch-activated ion channels in the membrane mediate tension-related electrical transduction”.
Recent experiments have shown that tension sensitivity is a property of ion channels other than those historically classified as “mechanosensitive” channels, and biophysicists are coming to see tension sensitivity as an intrinsic property of ion channels in general. However, efforts to elucidate the physiological relevance and molecular mechanisms of such tension sensitivity depend on the establishment of experimental methods that allow experimenters to evaluate dynamic changes in membrane tension in real time.
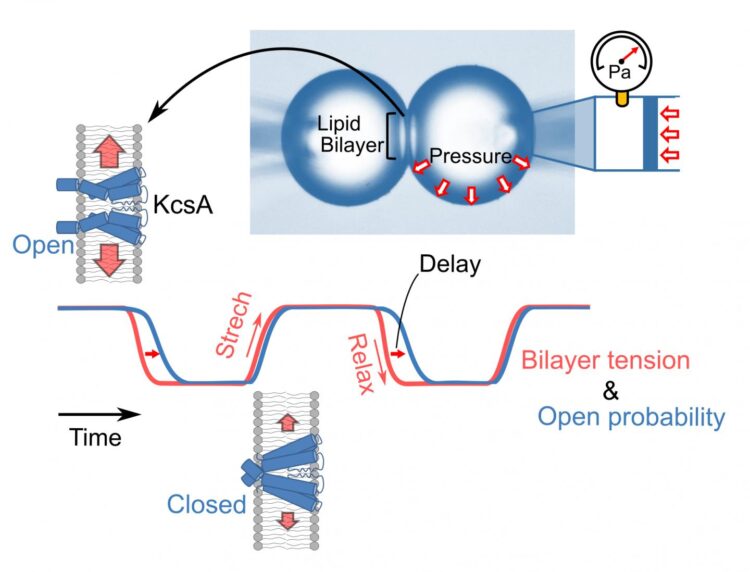
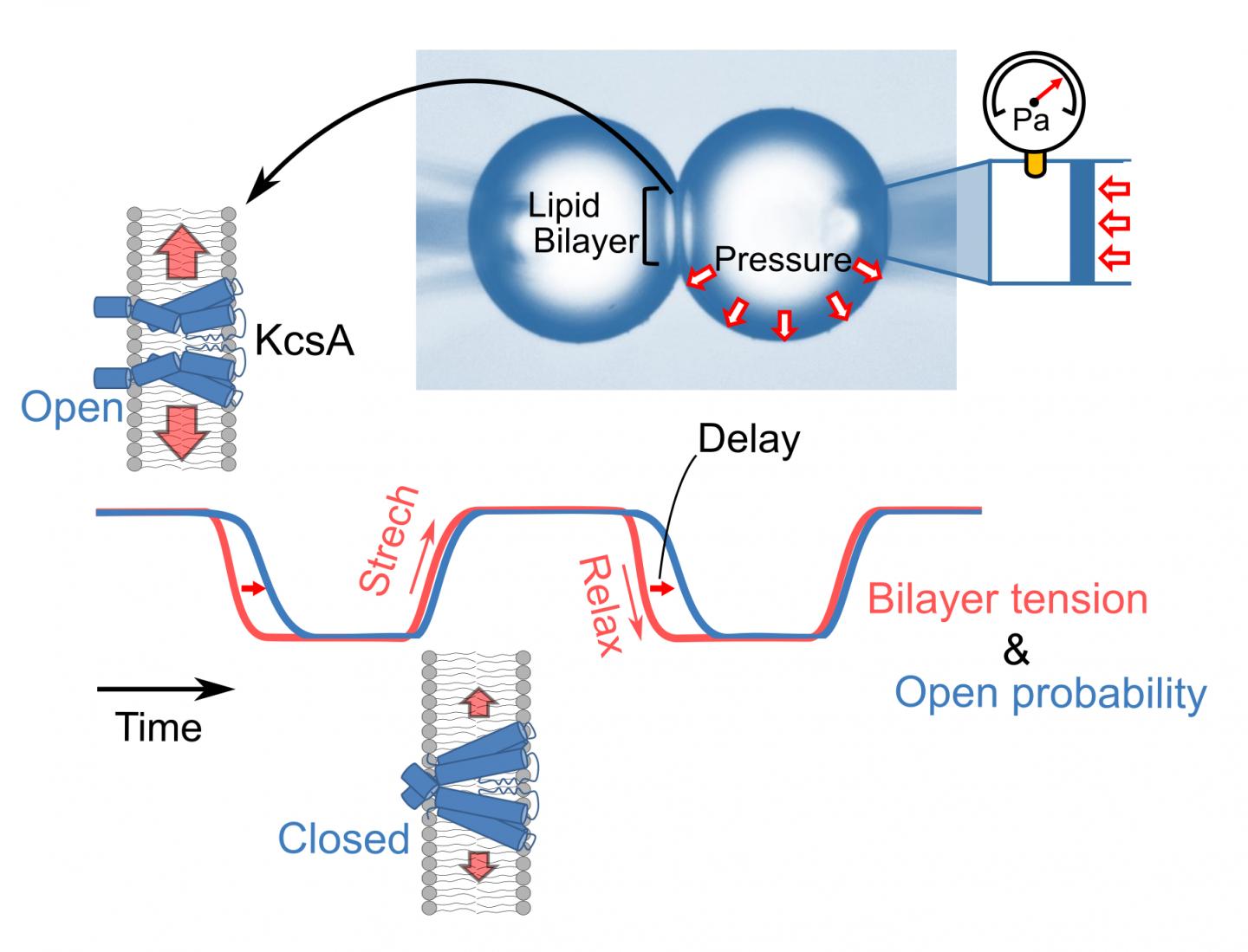
To meet this growing need, Professors Iwamoto and Oiki focused their efforts on developing a novel time-lapse system for measuring membrane tension. In their experiments, they formed a bilayer by docking two monolayer-lined water bubbles and evaluated tension using the Young-Laplace principle to measure intra-bubble pressures lower than 100 pascals. This novel experimental method has the advantage of relying on a straightforward model system consisting of purified channels and a simple lipid bilayer. This model allows experimenters to avoid the unmanageable complexity of real cell membranes, which feature a wide variety of ion channels and accessory proteins. The experimental setup permits real-time monitoring of membrane tension.
The KcsA ion channel is the prototypical ion channel used to understand ion channel structure-function relationships. The channel functions in the bilayer, and this is an important advantage given the rapid variability in membrane tension that occurs during actual cellular activity while recording the dynamic responsiveness of the KcsA ion channel. These experiments revealed a novel mode of action for tension sensitivity without precedent in the existing literature. Their results appear in a paper recently published in the peer-reviewed journal JACS Au.
Interestingly, the KcsA ion channels exhibited sensitivity to membrane tension and responded quickly to its fluctuations. One notable observation was that the ion channels’ responses to increasing membrane tension differed substantially from their responses to decreasing membrane tension. During the stretching phase, the channels started to activate only when the membrane tension reached high levels. In the tension decreasing phase, they remained active for a while even after returning to a low level of tension. This feature is called hysteresis, and it implies that the channel molecules can “memorize” their active state for a short period.
In conclusion, Professors Iwamoto and Oiki have developed a time-lapse system for measuring membrane tension while recording the dynamic responsiveness of a prototypical ion channel. Their findings revealed a process of hysteresis, which they note “extends existing knowledge of the mechanisms of the tension-sensitive channels that play key roles in various cellular activities.”
The present study is thus important both as a demonstration of a new method for fundamental ion channel research and as basic research into ion channel mechanisms. The insights into hysteresis as a functional feature of KcsA ion channels could be valuable for drug discovery research.
###
About University of Fukui, Japan
The University of Fukui is a preeminent research institution with robust undergraduate and graduate schools focusing on education, medical and science, engineering, and global and community studies. The university conducts cutting-edge research and strives to nurture human resources capable of contributing to society on the local, national, and global level.
Website: https:/
About Professor Shigetoshi Oiki from the University of Fukui, Japan
Professor Shigetoshi Oiki works within the Biomedical Imaging Research Center at the University of Fukui since 2019 as a Specially Appointed Professor. He worked as a Professor in University of Fukui Faculty of Medical Sciences between 1998 and 2019. He has authored numerous papers on the biophysics of ion channels.
About Professor Masayuki Iwamoto from the University of Fukui, Japan
Professor Masayuki Iwamoto works within the Division of Molecular Neuroscience in the Faculty of Medical Sciences at the University of Fukui. He has authored numerous papers on the subject of ion channels.
Funding information
This study was funded by KAKENHI grants from the Japan Society for the Promotion of Science.
Media Contact
Mika Hayashi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.