Researchers optimize a novel process for the efficient conversion of carbon emissions into useful chemicals like acetate using microbes
Credit: Pusan National University
Rapid global urbanization has dramatically changed the face of our planet, polluting our atmosphere with greenhouse gases and causing global warming. It is the need of the hour to control our activities and find more sustainable alternatives to preserve what remains of our planet for the generations to come.
Carbon dioxide (CO2) and carbon monoxide (CO) make up a large proportion of industrial flue gases. Recent research has shown that certain microorganisms are capable of metabolizing these gases into useful by-products. Thus, attempts are now being directed to using microbes to recycle these gases and convert them into useful chemicals in a process known as ‘carbon capture and utilization’ (CCU). This is a step beyond the current widespread practice of ‘carbon capture and storage’ (CCS). However, such CCU requires high energy input making the scaling up of this process difficult and expensive. How can this process then be optimized for maximum output?
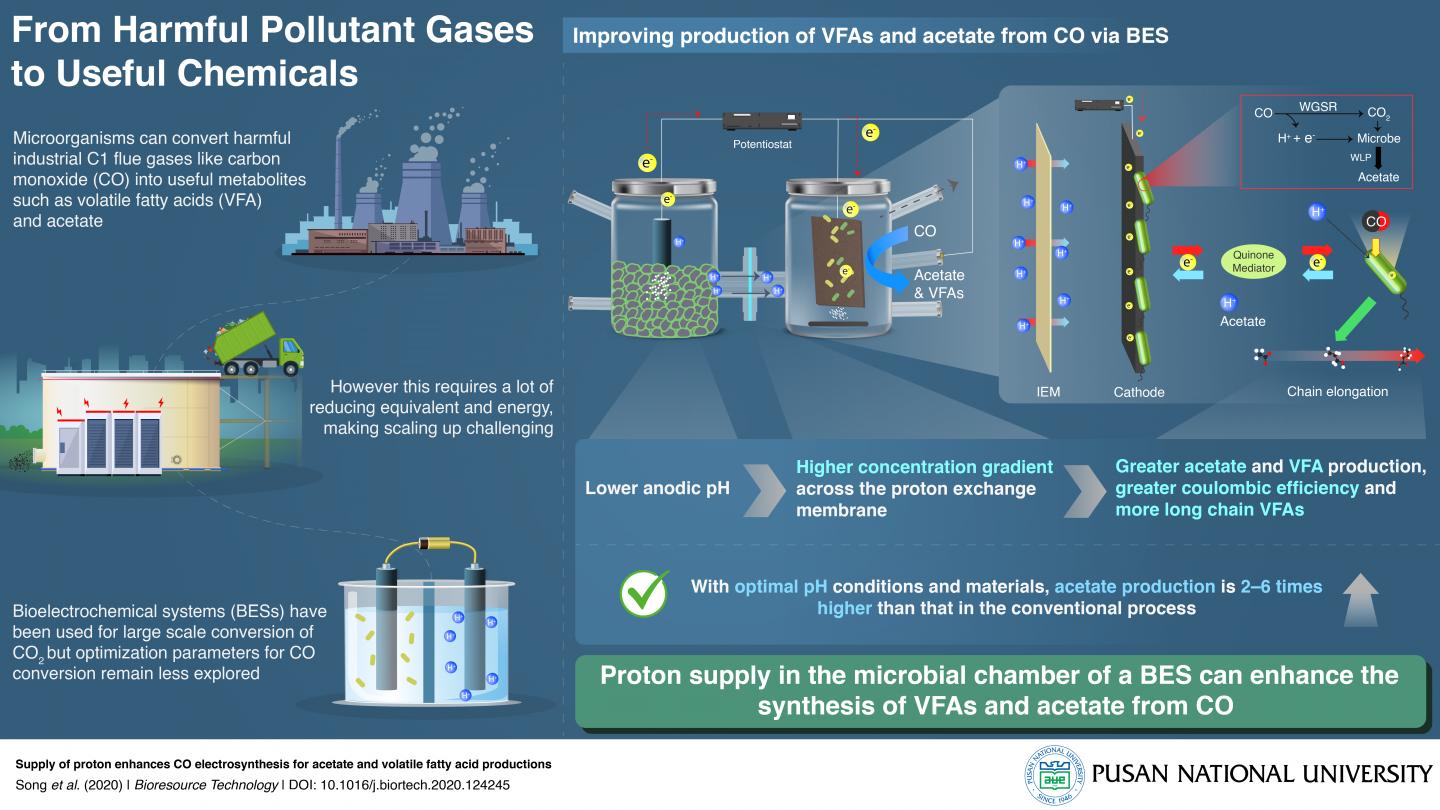
A team of researchers from Korea, led by Prof. Jung Rae Kim from Pusan National University, have answered this question for a newer CCU system called the bioelectrochemical system (BES). Prof. Kim explains, “We have developed a ‘bioelectrosynthetic process’ in which electroactive bacteria convert CO/CO2 into useful metabolites like acetate and volatile fatty acids using electricity as the reducing power.” The scientists were able to optimize BESs to increase their efficiency by 2 to 6 times that of current systems for CO gas. Their findings are published in Bioresource Technology since January 2021.
The two-chamber BES they used had several special features that achieved this. The cathode contained an electro-active biofilm, and the anode produced hydrogen ions via water electrolysis. These chambers were divided by an ion exchange membrane (IEM), which controlled the flow of protons and electrons between the chambers. Further, while the former contained microbial culture media, the latter contained mechanisms to control the initial pH of the system. In addition, a quinone electron mediator was used.
They found that, given the right IEM–one that allowed protons but not oxygen to pass through–an acidic pH in the anode chamber caused a higher proton concentration gradient across the membrane, which was key to enhancing acetate production and the synthesis of longer chain fatty acids in the cathode chamber. The quinone-dependent mediators improved electron transfer and increased product formation.
Prof. Kim states, “Since CO is a more reduced gas than CO2, perhaps unsurprisingly, the coulombic efficiency for CO was double that for CO2. CO is a major component in the industrial off-gas of most steel mill processes and biomass gasification. Via this BES conversion, it can be a valuable feedstock for various bioprocesses. This is the first study that makes the conversion of CO via BESs commercially viable.” Highlighting the applications further, he continues: “The microbes self-replicate, making this BES an economical solution. That combined with the efficiency we’ve achieved and the optimal system we’ve created should make this of sufficient interest to industries such that this becomes commercial industrial machinery within 5 years.”
This is one way in which the earth will be made cleaner, greener, and cooler!
###
Reference
Title of original paper: Supply of proton enhances CO electrosynthesis for acetate and volatile fatty acid productions
Journal: Bioresource Technology
DOI: 10.1016/j.biortech.2020.124245
About Pusan National University
Website: https:/
About Professor Jung Rae Kim
Dr. Jung Rae Kim is a Professor in the School of Chemical Engineering at Pusan National University.
Media Contact
Young-suk Hwang
[email protected]
Original Source
http://www.
Related Journal Article
http://dx.