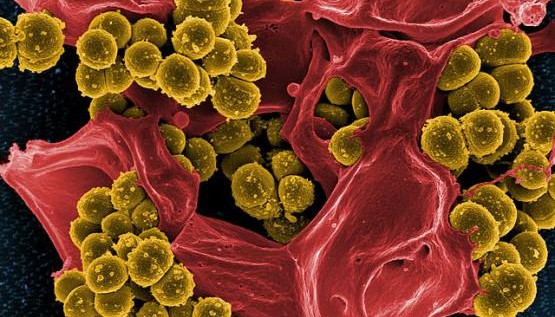
Researchers at the University of California, San Diego have developed a revolutionary new method for identifying and characterizing antibiotics, an advance that could lead to the discovery of new antibiotics to treat antibiotic resistant bacteria.
The researchers, who published their findings in this week’s early online edition of the journal Proceedings of the National Academy of Sciences, made their discovery by developing a way to perform the equivalent of an autopsy on bacterial cells.
“This will provide a powerful new tool for identifying compounds that kill bacteria and determining how they work,” said Joseph Pogliano, a professor of biology at UC San Diego who headed the research team. “Some bacteria have evolved resistance to every known class of antibiotic and, when these multi-drug resistant bacteria cause an infection, they are nearly impossible to treat. There is an urgent need for new antibiotics capable of treating infections caused by antibiotic resistant bacteria.”
The Centers for Disease Control and Prevention issued an alarming report in March that antibiotic-resistant strains of Carbapenem-Resistant Enterobacteriaceae, or CRE, had been found to cause infections in patients in nearly 200 hospitals in the United States alone. Because no antibiotics on the market are effective at treating these infections, about one-half of patients die from CRE infections. These outbreaks are difficult to contain, and in a 2011 outbreak of Klebsiella pneumonia at the U.S. National Institutes of Health Clinical Center, the bacteria spread despite strict infection control procedures and was detected in drains and medical devices that had been subject to standard decontamination protocols.
“We are finally running out of the miracle drugs,” said Pogliano, who detailed the history: The antibiotic penicillin was first discovered in the late 1920s, and received widespread clinical use in the 1940s. However, bacteria quickly evolved resistance to penicillin, so new and better versions were developed. Since that time, a continuous race has been fought to identify new antibiotics in order to stay one step ahead of the evolving resistance. In the 2011 outbreak of Klebsiella, the bacteria evolved resistance even to colistin, a drug of last resort because of its severe side effects.
Over the last 25 years, the number of new antibiotics entering the clinic has drastically declined. At the same time, bacteria have continued to evolve resistance to all of the currently available drugs, creating the current critical situation. One of the main problems in identifying new antibiotics and bringing them to market is a lack of understanding how the molecules work.
“It’s easy to identify thousands of molecules capable of killing bacteria,” explained Kit Pogliano, a professor of biology and a co-author of the paper. “The hard part is picking out the winners from the losers, and choosing molecules that are the best candidates for drug development. One key piece of information needed for this choice is knowledge of how the drug works, but this is traditionally difficult information to obtain, usually requiring months of intensive work. We’ve applied 21st century methods that within just two hours provide this information, allowing more rapid prioritization of new molecules. This will open up the discovery pipeline, allowing us to more rapidly identify new molecules with potential to enter the clinic for treatment of multi-drug-resistant pathogens.”
One key to this new approach was the combination of microscopy and quantitative biology tools. “We had to develop all of the cell biology and quantitative biology methods for generating the data ourselves and that required a lot of work, but now that we have the method working, it is very exciting,” said Poochit Nonejuie, a graduate student in the Division of Biological Sciences and another co-author. “My chemistry colleagues can give me a new molecule in the morning, and by the afternoon I can tell them the likely cellular pathways that they target. It’s mind blowing how powerful the technology is.”
The UC San Diego biologists say their new method is not only game changing, but promises to revolutionize how drug discovery teams guide their studies. With previous methods, understanding how an antibiotic works requires many different biochemical assays to be performed, which requires a lot of time and relatively large quantities of the compound, which is almost always in short supply when it is first discovered.
“Our new method represents the first time that a single test can be performed and identify the likely mechanism of action for a new compound,” said Joseph Pogliano. He noted that postdoctoral fellow Anne Lamsa has miniaturized the method so that it requires just a few nanograms of each drug candidate, conserving molecules that are often available only in tiny quantities.
“It’s also faster and can be easily adapted for high-throughput drug discovery efforts,” he added. “This method will allow us to more quickly identify chemicals that kill bacteria, which will accelerate the development of new medicines. Understanding how antibiotics work is key to understanding how they evolve resistance.”
Pogliano said his research team, which also included Mike Burkart, a chemistry and biochemistry professor, will be continuing its investigations on antibiotics. “We are now using this method to look for new molecules active against antibiotic resistant bacteria,” he said.
The study was funded by the National Institutes of Health (NIH grants AI095125 and GM073898).
Story Source:
The above story is based on materials provided by University of California – San Diego.