Credit: Yujiro Hoshino, Yokohama National University
A team of researchers from Japan has demonstrated a light-based reaction that yields high numbers of the base chemical component required to produce bioactive compounds used in common industry products.
They published their results on June 11 in Organic Letters.
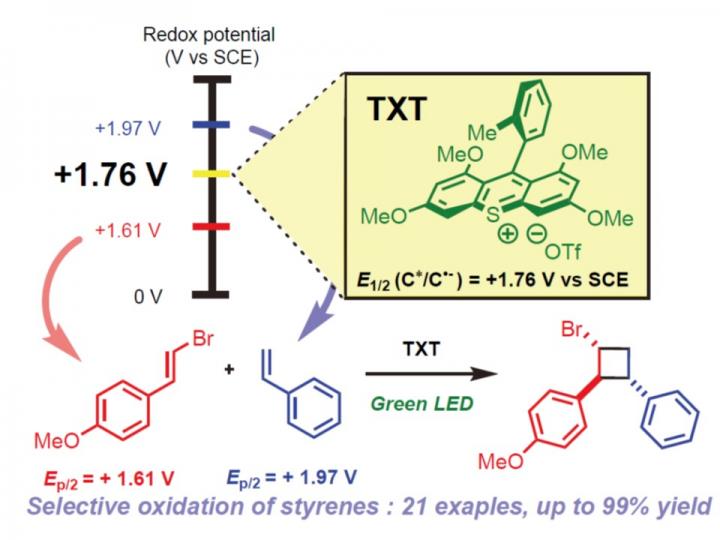
“We developed a redox potential-controlled and cost-effective method to synthesize multisubstituted cyclobutanes, which are present in the core structure of various products and bioactive components,” said paper author Yujiro Hoshino, a research associate at Yokohama National University.
Cycloaddition reactions allow to prepare carbocyclic and heterocyclic organic compounds with atom-efficiency. For a long time, researchers carried out photocycloadditions of olefins such as styrenes, a chemical used in the production of plastics and rubber, by treating them with high-energy ultraviolet light or transition metal catalysts, which are known to be toxic and expensive chemical reagents. The reaction mainly provides homo-dimer, not hetero-dimer. In addition, the powerful light damages the bonds holding the chemical together, allowing it to break apart and reform in a new configuration, known as a cyclobutene ring.
Redox potential refers to how easily a chemical loses or gains electrons. Hoshino and co-workers take advantage of this characteristic and applied a green, visible light to styrenes situated in a two-by-two arrangement, allowing the chemical components and bonds to selectively reorganize as the light freed electrons from the styrenes. The newly organized chemical components were multisubstituted cyclobutanes.
“By focusing on the different redox potential between various styrenes and optimizing our light catalysts, we developed a mild and clean method to synthesize multisubstituted cyclobutanes,” Kenta Tanaka, paper first author and an assistant professor at Tokyo University of Science, said.
“Emphasis will be placed on the strategy which shows the potential to synthesize multisubstituted cyclobutanes via radical cation species without any transition metal catalysts,” said another corresponding author Kiyoshi Honda, a professor at Yokohama National University.
Next, the researchers plan to expand the use of various visible-light catalysis methods.
“We hope our reaction system provides an efficient and new method for green-light-driven organic chemical reactions, and that we continue to contribute to the field,” Hoshino said.
###
This work was supported in part by the Nanotechnology Platform Program at the Ministry of Education, Culture, Sports, Science and Technology in Japan and Yokohama National University.
Other contributors include Yoshinori Iwama and Mami Kishimoto, both of whom are affiliated with the Graduate School of Environment and Information Sciences at Yokohama National University; and Naoya Ohtuska, Institute for Molecular Science.
Yokohama National University (YNU or Yokokoku) is a Japanese national university founded in 1949. YNU provides students with a practical education utilizing the wide expertise of its faculty and facilitates engagement with the global community. YNU’s strength in the academic research of practical application sciences leads to high-impact publications and contributes to international scientific research and the global society. For more information, please see: https:/
Media Contact
Akiko Tsumura
[email protected]
Related Journal Article
http://dx.




