New research from UT Southwestern may help those with rare muscle diseases
Credit: UTSW
DALLAS – May 25, 2020 – Scientists have known for a decade that cells that fuse with others to perform their essential functions – such as muscle cells that join together to make fibers – form long projections that invade the territory of their fusion partners. But how the thin and floppy polymers involved in this process propel mechanically stiff protrusions has been unknown.
In a new study published online today in Nature Cell Biology, UT Southwestern scientists outline the mechanisms behind the formation of these projections, focusing on the interaction between two proteins known as actin and dynamin. The findings, they say, offer insight on a key cellular process that’s essential for the conception, development, regeneration, and physiology of multicellular organisms and may eventually lead to new treatments for a rare muscle disease.
Cell fusion involves three main steps, explains study leader Elizabeth Chen, Ph.D., a professor in the departments of molecular biology and cell biology at UT Southwestern Medical Center whose research focuses on this process. First, adhesion molecules draw cell membranes together, but leave a gap between cells; next, one cell extends fingerlike projections that invade the other cell; finally, so-called fusogenic proteins bring the cells’ membranes even closer to touch and merge.
For that middle step, Chen says, research from her lab and others has shown that a protein called actin plays a key role in forming projections. However, actin forms floppy and thin polymers, known as actin filaments, each with a diameter of only 7 nanometers. How these thin filaments become mechanically rigid enough to push out projections that invade other cells was unclear.
To solve it, Chen and her colleagues studied actin’s interaction with dynamin, a protein that can release energy from specific chemical bonds found throughout cells. One of dynamin’s roles is to pinch off newly formed vesicles that bring cargo into cells, by forming a structure around the “neck” of the vesicles protruding in from the cell membrane. Although previous studies have shown that dynamin and actin were associated with each other in many cellular structures, how they work together has remained a mystery for two decades.
Using fruit fly muscle cells as a model system, Chen and her team started by observing muscle cell fusion in embryos genetically engineered to not make any functional dynamin. They found that without dynamin function, not only could these cells no longer merge, they also couldn’t form the normal projections, suggesting that dynamin plays a key role in this step of the process.
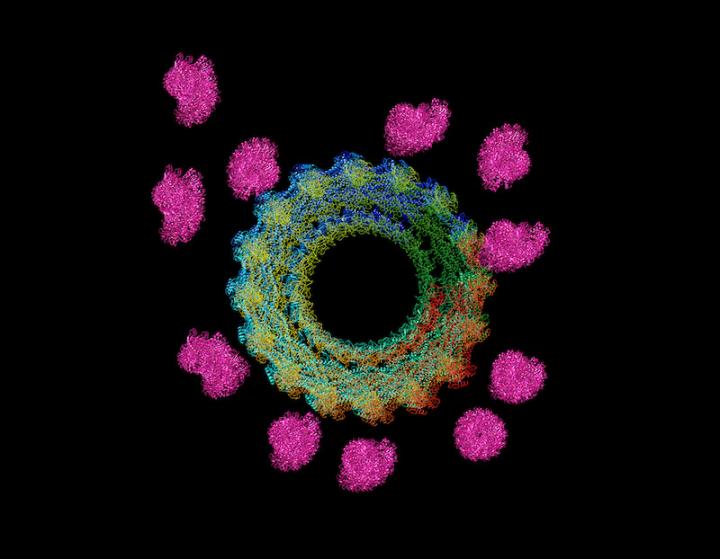
The researchers then used microscopy to take a closer look at how purified dynamin and actin proteins interacted when they were mixed in equal parts. They found that the actin filaments appeared to be organized into bundles held together at regularly spaced intervals by dynamin, the latter of which forms a helix when multiple dynamin molecules assemble together. Surprisingly, rather than the dynamin helix wrapping around the actin filaments, the filaments bind to the outer rim of the helix, with each helix capturing up to 16 filaments.
Although this experiment shows that dynamin has the capacity to capture and hold multiple actin filaments into stronger bundles, Chen says, fully occupied dynamin helices are unlikely to last long in cells, where ample energy sources that can cause these dynamin structures to dissolve into individual units is abundant. Sure enough, when the researchers added energy sources to the dynamin-actin mix, the dynamin helices did come apart, but not in a synchronized fashion. While fully assembled helices broke apart, others remained – keeping the actin bundles together while allowing new filaments to emanate from areas unbound by dynamin. Such a dynamic process ultimately leads to the formation of multiple interconnected parallel actin bundles, hence further increasing the mechanical strength of the actin network, says Chen. Experiments in cells showed that the dynamic actin bundling process was critical for cells to form projections and fuse with other cells.
Although Chen and her colleagues used muscle cells as their model system, Chen notes that the interplay between actin and dynamin they discovered here could play a key part in other types of cell fusion, such as between bone-resorbing cells or between immune cells. Defects in this process could also be responsible for some rare disorders such as centronuclear myopathy, a condition in which muscle cells form fibers that are too small. Previous research has shown that multiple genetic mutations in dynamin can cause this disease.
“We are interested in looking at how the human mutations are blocking the fusion process, which could eventually lead to novel ways to intervene and help these patients,” Chen says.
###
Other UTSW scientists who contributed to this research include Ruihui Zhang, Donghoon M. Lee, Marcel Mettlen, Michael E. Abrams, Peter Keene, Pratima Pandey, Benjamin Ravaux, Jonathon A. Ditlev, Michael K. Rosen, Neal M. Alto, and Sandra L. Schmid.
This work was supported by several National Institutes of Health grants (R01 AR053173, R01 GM098816, R01 GM095977, R01 GM42455, R01 GM104032, R01 AI083359, and R01 GM127673); an American Heart Association Established Investigator Award; a Howard Hughes Medical Institute (HHMI) Faculty Scholar Award; Welch Foundation grants (I-1823 and I-1704); an HHMI and Simons Foundation grant; a Chan Zuckerberg Biohub Investigator Award; and an HHMI Faculty Scholar Award.
About UT Southwestern Medical Center
UT Southwestern, one of the premier academic medical centers in the nation, integrates pioneering biomedical research with exceptional clinical care and education. The institution’s faculty has received six Nobel Prizes, and includes 25 members of the National Academy of Sciences, 16 members of the National Academy of Medicine, and 14 Howard Hughes Medical Institute Investigators. The full-time faculty of more than 2,500 is responsible for groundbreaking medical advances and is committed to translating science-driven research quickly to new clinical treatments. UT Southwestern physicians provide care in about 80 specialties to more than 105,000 hospitalized patients, nearly 370,000 emergency room cases, and oversee approximately 3 million outpatient visits a year.
Media Contact
Newsroom
[email protected]
Original Source
https:/




