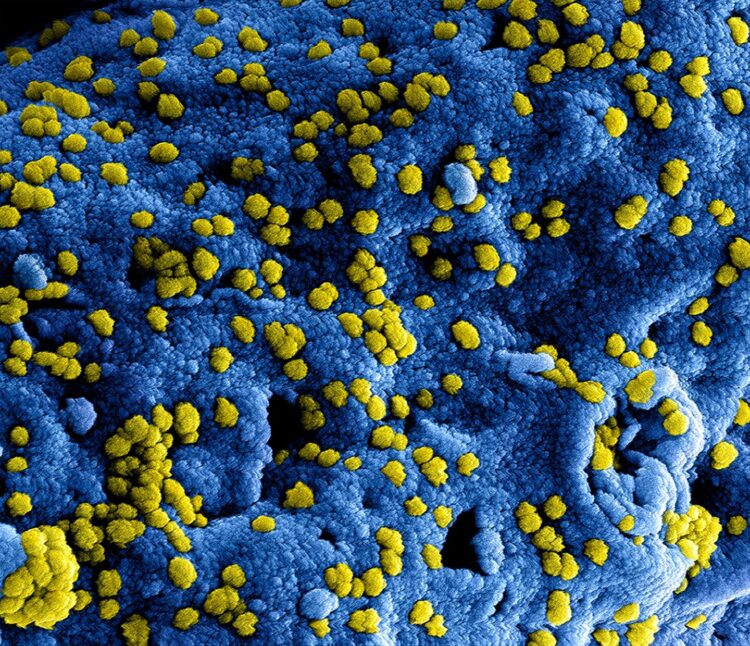
Insights may be useful for the development of a vaccine against the new coronavirus
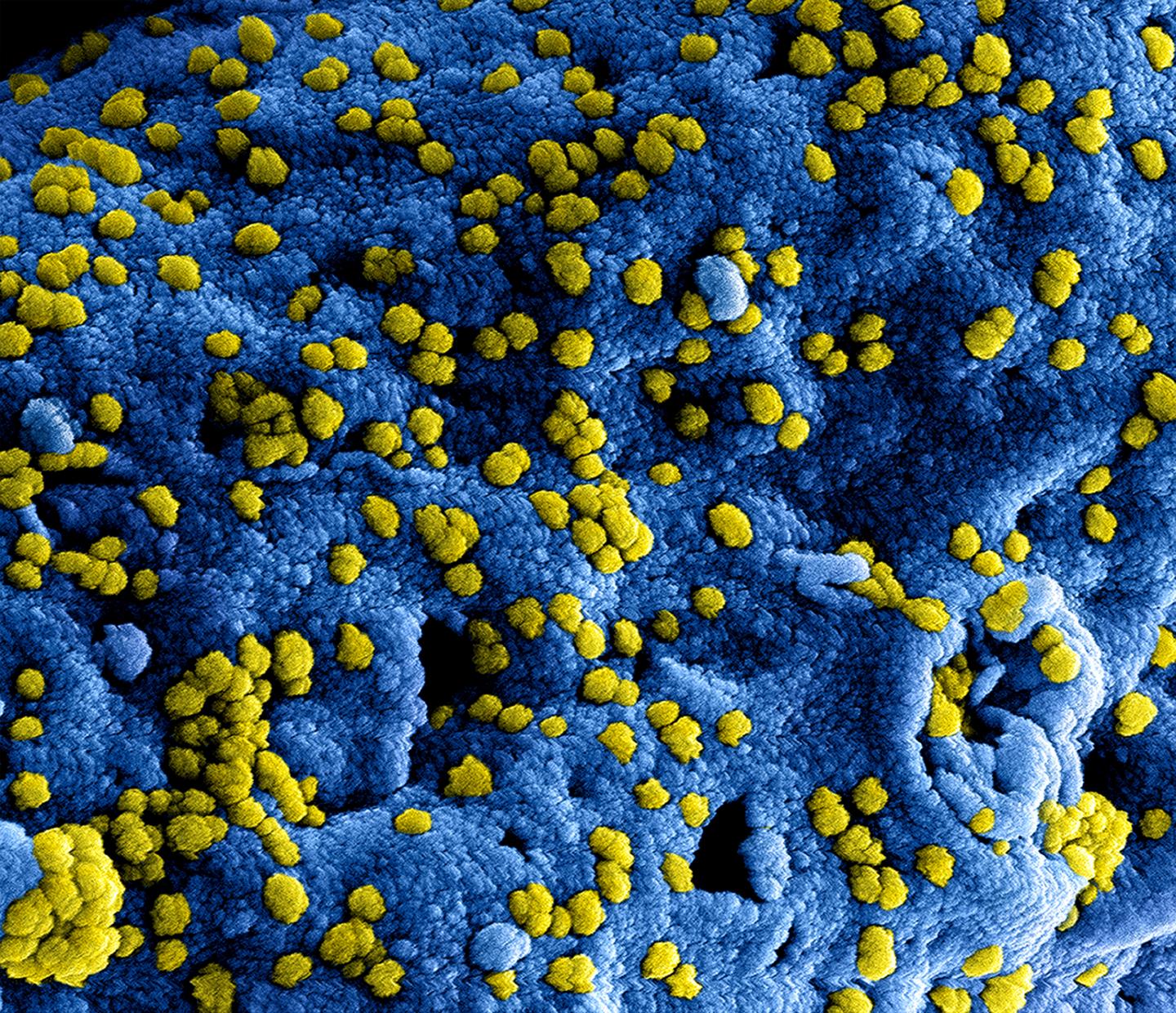
Credit: NIAID
“The results of this vaccine trial are also important and promising with regard to the development of a vaccine against SARS-CoV-2, the new coronavirus,” explains Prof. Marylyn Addo, Head of the Division of Infectious Diseases at the UKE and scientist at the DZIF. “The development of the MERS vaccine provides a basis upon which we at the DZIF can rapidly develop a vaccine against the new coronavirus.”
The MERS coronavirus, identified for the first time in 2012, is listed on the World Health Organisation´s Blueprint list for pathogens that are considered a particular threat to public health. The virus is transmitted from dromedary camels to humans and is also transmissible between humans. Infections with the virus cause respiratory illness with a mortality of up to 35 percent. Worldwide, close to 2,500 cases of MERS have been detected in 27 countries with the highest numbers being in Saudi Arabia. To date, neither an effective vaccine against the MERS coronavirus nor a specific drug exist.
The vaccine candidate MVA-MERS-S
“In 2014, together with DZIF partners, we started to develop a vaccine against the MERS coronavirus in preparation for larger outbreaks of the virus in the future,” Addo explains. The vaccine is based on an attenuated virus (MVA: modified vaccinia virus Ankara), which had previously been used in a smallpox eradication vaccination campaign and has now been altered to contain protein components from the MERS coronavirus. This recombinant, so-called vector-based vaccine, scientifically termed MVA-MERS-S for short, is to boost immunity against MERS coronaviruses. Prof. Gerd Sutter from Ludwig-Maximilians University of Munich developed this vaccine in collaboration with Philipps University of Marburg and the Erasmus Medical Center Rotterdam. The MVA vector now serves as a basis for developing a vaccine against SARS-CoV-2, the new coronavirus.
The vaccine trial
The vaccine trial was conducted in collaboration with the Clinical Trial Center North (CTC North). A total of 23 healthy trial volunteers were vaccinated twice with MVA-MERS-S, the experimental vaccine, with an interval of four weeks between the vaccinations. The trial was to answer two questions: Is the experimental vaccine MVA-MERS-S tolerated well and safe to use in humans? Does it trigger humoral and cellular immune responses in humans, i.e. a development of antibodies and T cells that are able to prevent MERS-CoV infection or curb the course of illness?
The most important findings
“The tolerability and safety of the vaccine candidate as well as the resulting immune responses are very promising,” explains Dr Till Koch, one of the first authors of the trial and a DZIF stipend-holder. The vaccine was well tolerated. Local side effects (i.e. pain at the site of injection, mild erythema and warmth) occurred most frequently and presented in 69 percent of the trial subjects. No severe side effects occurred. “After the second injection of MVA-MERS-S, antibody formation and T cell responses occurred in 87 percent of the trial subjects,” summarises first co-author Dr Christine Dahlke.
Prof. Stephan Becker is pleased, “These results show that the new vaccine could potentially be used in future MERS outbreaks.” The trial subjects’ antibody responses were investigated in his laboratory at the University of Marburg. At the DZIF, Stephan Becker coordinates the research area “Emerging Infections” and is substantially involved in all vaccine projects.
The path to a vaccine
Next, a phase Ib trial, funded by CEPI (Coalition for Epidemic preparedness Innovation), will be conducted in which the vaccine will be tested in 160 trial subjects in Hamburg and Rotterdam. At the German Center for Infection Research, the results and tests from this trial will be used to start the development of a vaccine against the new coronavirus as rapidly as possible. The scientists will use the same viral vector (MVA) into which they will insert a SARS-CoV-2 spike protein to replace the MERS-CoV spike protein.
###
Media Contact
Marylyn Addo
[email protected]
Original Source
https:/
Related Journal Article
http://dx.