Novel vaccine delivery technology will contribute to efforts to develop universal vaccines and improve global immunization capabilities essential for combating coronavirus, reports the Journal of Investigative Dermatology
Credit: UPMC
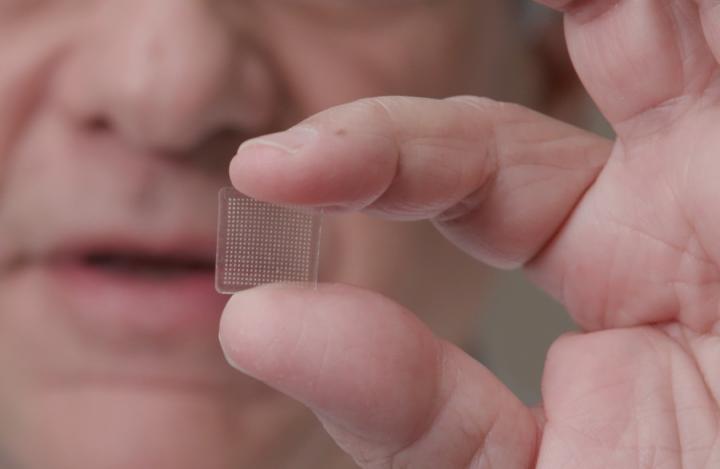
Philadelphia, April 21, 2020 – In parallel to their current work on a potential coronavirus vaccine, researchers at the University of Pittsburgh School of Medicine have developed a new vaccine delivery system for vaccines using live or attenuated viral vectors: a finger-tip sized patch that contains 400 tiny needles, each just half of one millimeter. Their progress is reported in the Journal of Investigative Dermatology, published by Elsevier.
The needles, made from sugar and the specific cargo being delivered, comprise a three-dimensional (3D), multicomponent dissolving microneedle array (MNA). While feeling like having Velcro pressed against the skin, the vaccine would penetrate the upper level of the skin, absorb moisture from the skin, and then dissolve and release molecules that prompt the immune system to make antibodies to attack the virus. In addition to antibody production, this technology has the potential to improve cellular immune responses in patients and expand global immunization capabilities. It is clear evidence of the broad reach and contribution of skin scientists, even extending into pandemic vaccine design.
Explaining the importance of this work, lead author Louis D. Falo, Jr., MD, PhD, Professor and Chairman of the Department of Dermatology, University of Pittsburgh School of Medicine and UPMC, Pittsburgh, PA, USA, explains, “We are developing this new delivery technology because while traditional vaccines are often effective in inducing antibody responses, they frequently fail to generate the cellular responses that are essential to prevent or treat many cancers or infectious diseases.”
The skin is an ideal vaccination site because it contains an immune network that is highly responsive and encourages the generation of strong and long-lasting immunity.
Dissolvable MNAs are designed to mechanically penetrate the superficial cutaneous layers, rapidly dissolve upon insertion into the skin, and deliver uniform quantities of biocargo to a defined 3D space within the skin. This enables localized delivery of low amounts of drugs or vaccines to achieve high concentrations in this specific skin microenvironment.
Using in vivo mouse models, investigators generated the 3D multicomponent dissolvable vaccine platform combining a live adenovirus-encoded antigen with an added component, polyinosinic:polycytidylic acid (poly I:C), an immunostimulant used to simulate the skin immune system. This successfully induced both antibody responses and stronger cellular immune responses.
Induction of antigen-specific cellular immunity is a point of emphasis in the vaccine field, as evidenced by recent efforts to generate “universal vaccines” for mutable infectious diseases like influenza, HIV, and coronaviruses by targeting infected cells.
“Remarkably,” says Dr. Falo, “the MNA vaccine platforms incorporating both antigen-encoding adenovirus and poly I:C augmented the destruction of targeted cells significantly compared to MNA-delivery of the same adenovirus alone.” The researchers also found that the MNAs integrating both poly I:C and adenovirus retained their immunogenicity after one month of storage at 4? C. MNA-delivered vaccines also have advantages in their ease of fabrication, application, and storage compared to other vaccine delivery platforms.
“Our results suggest that multicomponent MNA vaccine platforms uniquely enable delivery of both adjuvant and antigen-encoding viral vectors to the same skin microenvironment, resulting in improved immunogenicity including cellular immune responses,” comments Dr. Falo. “This MNA delivery approach could improve the effectiveness of adenoviral vaccines now in development for the prevention of coronavirus disease (COVID-19).”
###
Media Contact
Theresa Monturano
[email protected]
Related Journal Article
http://dx.




