A joint research group at Osaka University and the University of Tokyo discovered how the electrons in a magnetic pyrochlore oxide spontaneously freeze into a ‘glassy state’ at low temperatures without reference to an extrinsic disorder
Credit: Osaka University
Osaka, Japan – A joint research group from Osaka University and the University of Tokyo uncovered the mechanism of the glass transition that electrons can experience in pyrochlore oxide crystals. The researchers show that distortions in the atomic lattice cause two types of rotational degrees of freedom of spins to become coupled and form a glassy state at the exact same temperature. This work will shed light on our understanding of the mechanism of glass transitions, which is one of the most fundamental unsolved problems in physics.
Pyrochlore oxides are minerals that have the chemical formula A2B2O7, where A is usually a rare earth ion and B is a transition metal–in this case, molybdenum. The metal ions in the crystal form tetrahedra that share corners. The electrons in the ions are essentially bound to the nucleus but they can still orbit around the nucleus and spin around themselves. In a sense, this is similar to motions of planets in the solar system: planets are orbiting around the sun while also spinning around themselves.
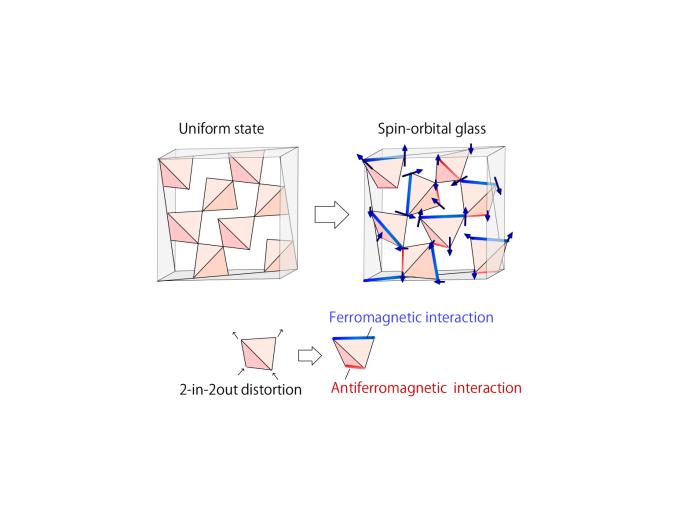
Scientists found that the orbits and spins of the electrons on different corners of the tetrahedra are interacting with each other in a complex way. Some pairs of spins want to align their axes of spin in parallel but others want to align anti-parallel. Unfortunately, there is no possible way to meet all of these simultaneously, so scientists say that the spins are “frustrated.” The result is many equivalent configurations and the spins end up stuck pointing in different directions even at low temperatures. This is known as a spin-glass, since it has very similar dynamics to the cooling of molten glass to solid state. That is, the glass we are used to in our windows and cups is in an intermediate state between solid and liquid. The molecules are fixed in place, like a solid–since they don’t have enough energy to move–but they are arranged without long-range order, somewhat like a “frozen liquid.”
“Although some systems are known to show such behaviors due to extrinsic randomness, called ‘quenched disorder,’ we have shown that this is not needed to understand the glassiness of the pyrochlore system,” says first author Kota Mitsumoto.
While nature often appears to favor symmetric forms, there are cases in which tetrahedral crystals are more stable when one side is elongated and another is compressed, in a process called the Jahn-Teller distortion. The researchers found that this change coupled the spin and orbital degrees of freedom, which made them undergo glass transitions at the same critical temperature. “We were happy to be able to help solve a long-standing puzzle on the origin of the disorder-free spin glass,” adds senior author Hajime Yoshino.
The team used computer simulations along with theoretical calculations to show that, at this critical temperature, the non-linear response to external magnetic fields becomes very large, as expected for a glass transition.
“We demonstrated, for the first time, how a thermodynamic glass transition can occur on a periodic lattice without quenched randomness,” says Mitsumoto. “We hope that our findings can improve the understanding of the glass transition in general.”
###
The article, “Spin-orbital glass transition in a model frustrated pyrochlore magnet without quenched disorder” was published in Physical Review Letters at DOI: https:/
About Osaka University
Osaka University was founded in 1931 as one of the seven imperial universities of Japan and now has expanded to one of Japan’s leading comprehensive universities. The University has now embarked on open research revolution from a position as Japan’s most innovative university and among the most innovative institutions in the world according to Reuters 2015 Top 100 Innovative Universities and the Nature Index Innovation 2017. The university’s ability to innovate from the stage of fundamental research through the creation of useful technology with economic impact stems from its broad disciplinary spectrum.
Website: https:/
Media Contact
Saori Obayashi
[email protected]
81-661-055-886
Original Source
https:/
Related Journal Article
http://dx.




